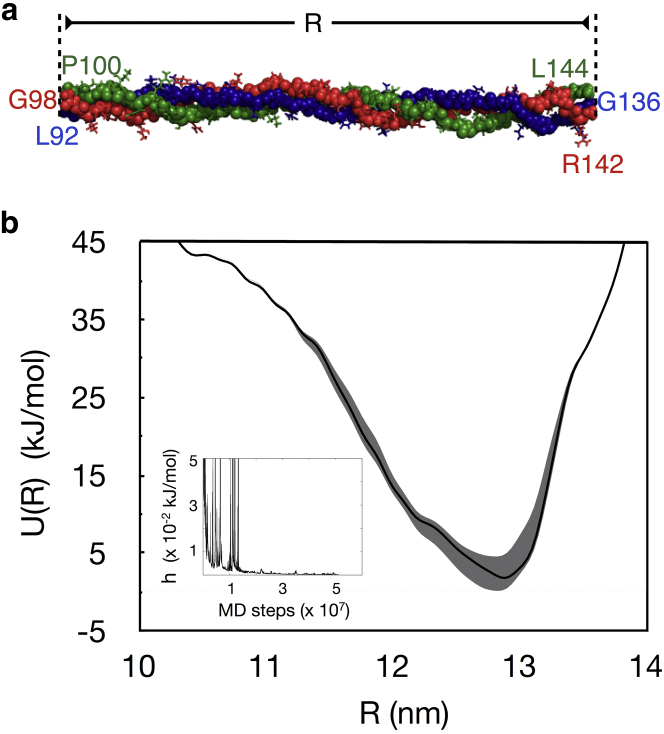
Figure 2.
(a) Representative 45-residue fragment of the collagen molecule. The backbone atoms are drawn as spheres and the side chains are drawn as sticks. The primary sequences of the two α1 chains are and , and the primary sequence of the α2 chain is . The h superscript over selected residues refers to their posttranslationally modified hydroxylated forms, and the subscript on the terminal residues of the chains matches their specific numbers in the primary sequence taken from the fiber diffraction structure of the fibril (PDB: 3HR2). Note that the residue numbers in the three chains are different, and this is because the three chains in the triple helix are staggered. (b) Potential of mean force (U(R)) of this fragment evaluated as a function of its end-to-end distance (R) from well-tempered metadynamics. (Solid line) Estimate from a trajectory comprising 5 × 107 MD steps (100 ns of metadynamics time). (Shaded area) Range bounded by two estimates, one from a smaller trajectory comprising 4.5 × 107 MD steps and the other from a longer trajectory comprising of 5.5 × 107 MD steps. (Inset) Time evolution of the dynamic hill height (h). To see this figure in color, go online.