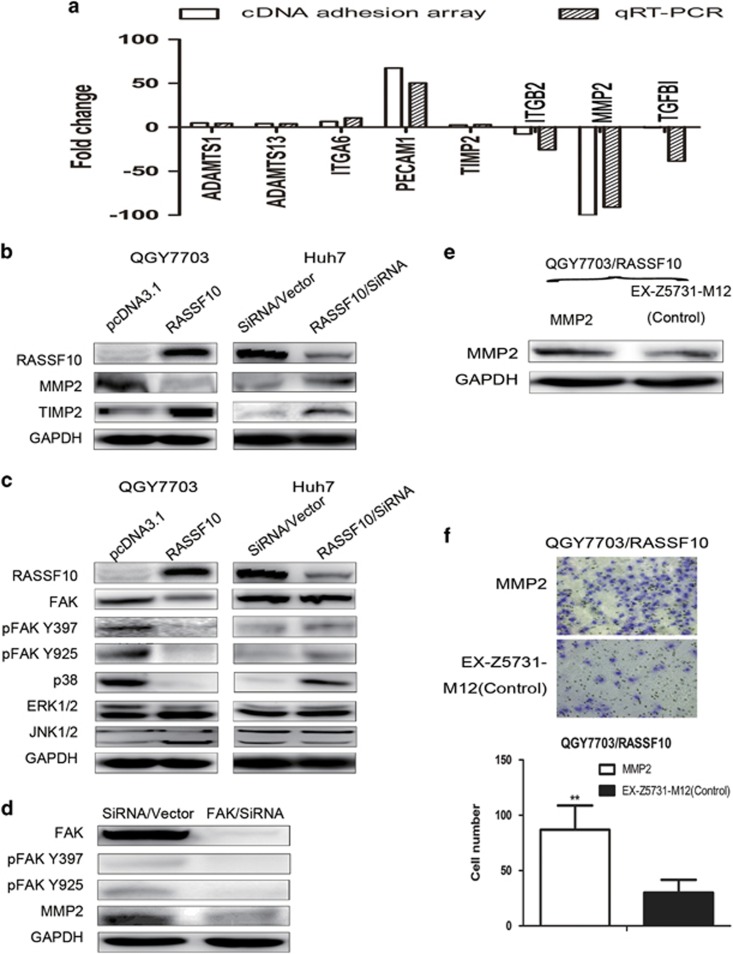
Figure 5.
RASSF10 impaired cell adhesion through MMP2 via FAK signaling. (a) Eight genes modulated by RASSF10 in HCC migration or invasion were found by cDNA adhesion array, which were further confirmed by qRT-PCR. White bars indicate the result of cDNA adhesion array, and black bars represent qRT-PCR data (the value of 2−ΔΔCT) in QGY 7703 transfected with pcDNA3.1/RASSF10 or empty vector (pcDNA3.1). (b) Western blot confirmed the association between RASSF10 and MMP2 or TIMP2 by over-expression or knock-down assay. (c) Regulatory effect of RASSF10 on FAK and MAPKs. Over-expression of RASSF10 has suppressed the accumulation of total or phosphorylation FAK and p38 MAPK; consistently, down-regulation of RASSF10 by SiRNA/RASSF10 in Huh 7 cells induced the activity of FAK, as well as p38 MAPK. The expression of ERK1/2 or JNK1/2 was independent on the level of RASSF10. (d) Regulatory effect of FAK on MMP2. Depletion of FAK suppressed the expression of MMP2. (e) Rescued assay for MMP2 in stable cell lines (QGY7703/RASSF10) was evidenced by western blot. (f) The change of cell invasion property was re-evaluated following MMP2 rescued assay. More invaded cells were observed with the restored expression of MMP2 in QGY7703/RASSF10. Invaded cells were stained with cell stain solution, counted by microscope in five random high power fields. Data are mean±s.d. The asterisk indicates statistical significance (*P<0.05, **P<0.01).