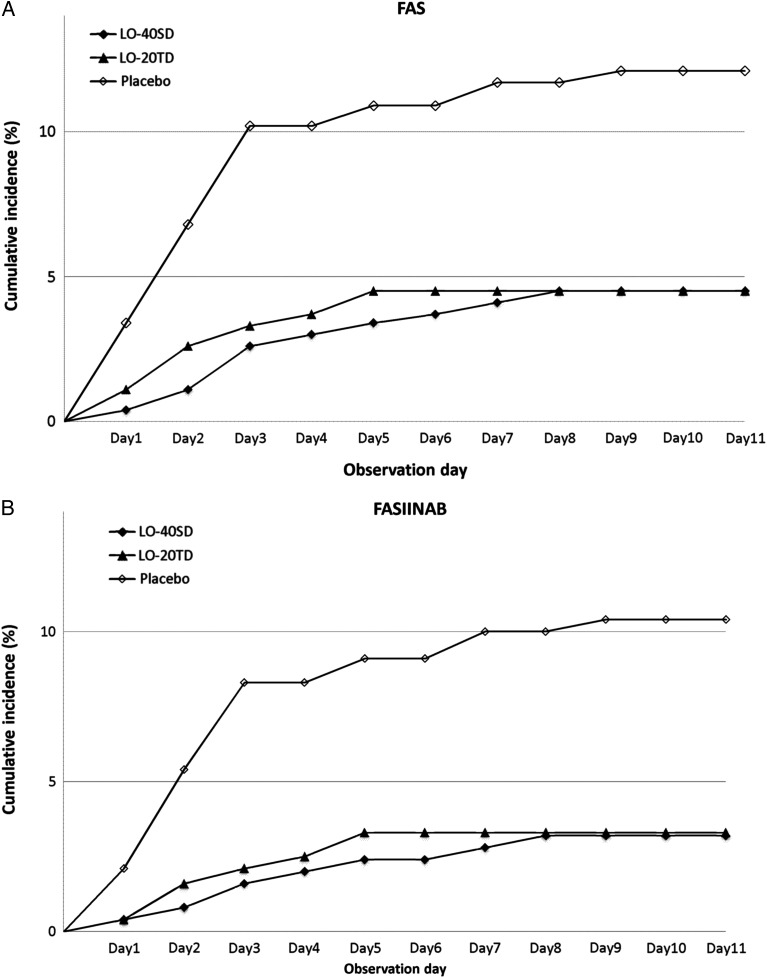
Figure 2.
Cumulative number of participants with clinical influenza, the primary endpoint, according to observation day. A, The cumulative number of participants with clinical influenza, the primary endpoint, by observation day in the full analysis set (FAS). B, The cumulative number of participants with clinical influenza, the primary endpoint, by observation day in the FAS index-infected virus-negative at baseline set (FASIINAB). Abbreviations: LO-20TD, 20 mg of laninamivir octanoate administered once daily for 2 days; LO-40SD, 40 mg of laninamivir octanoate, single administration.