Abstract
Pancreatic islets are heterogeneous clusters mainly composed of α and β cells, and these clusters range in diameter from 50 to several hundred micrometers. Native small islets are known to have a higher insulin secretion ability in vitro and to provide better transplantation outcomes when compared with large islets. In this study, we prepared microengineered pseudo-islets from dispersed rat islet cells using precisely-fabricated agarose gel-based microwells with different diameters (100, 300, or 500 μm) to investigate the function and survival of islet cell aggregates with well-controlled sizes. We observed that dead cells were rarely present in the small pseudo-islets with an average diameter of ∼60 μm prepared using 100 μm microwells. In contrast, we observed more dead cells in the larger pseudo-islets prepared using 300 and 500 μm microwells. The relative amount of hypoxic cells was significantly low in the small pseudo-islets whereas a hypoxic condition was present in the core region of the larger pseudo-islets. In addition, we found that the small-sized pseudo-islets reconstituted the in vivo-tissue like arrangement of the α and β cells, and restored the high insulin secretory capacity in response to high glucose. These results clearly suggest that precise size control of pseudo-islets is essential for maintaining islet cell function and survival in vitro. The small-sized pseudo-islets may be advantageous for providing a better therapeutic approach for treating type 1 diabetes mellitus via islet reorganization and transplantation.
Keywords: Medicine, Engineering, Bioengineering
1. Introduction
Type 1 diabetes is an autoimmune disease involving destruction of β cells in the pancreatic islets of Langerhans. Type 1 diabetic patients need to take insulin by injection for their entire life. Recently, pancreatic islet transplantation has been developed as an alternative therapy to insulin injection for patients with severe type 1 diabetes [1]. Islet transplantation is less invasive when compared to transplanting the whole pancreas. Isolated islets are transplanted into the liver through the portal vein in most cases, but the procedure requires a large amount of islets, usually from multiple (2–3) donors for treating one patient. Additionally, islet transplantations often need to be done multiple times to achieve insulin independence, because of graft damage and cell loss in the pre- and post-transplant period. Recently, a single-donor islet transplant has been reported [2, 3], but it is possible only at selected facilities and thus, is not universally available for all patients [4].
Another problem with the current islet transplantation therapy is that the long-term outcomes of insulin independence are poor when compared with that of whole pancreas transplantation. It was reported that about 70% of the patients transplanted with islets using the original Edmonton Protocol maintained insulin independence at 1 year post-transplantation, but it decreased to only 7.5% after 5 years [5]. More recently, the rate of five-year insulin independence has improved with advanced methods, but it is still only approximately 50% [6]. The current challenge of islet transplantation is to provide long-term insulin independence using lower islet masses from a single donor. From this viewpoint, an improvement in islet cell function and survival is needed for developing more efficient pancreatic islet transplantation.
It is well known that there is a size distribution of native islets in the pancreas ranging from 50 to 500 μm [7, 8]. Many studies have suggested that there is a close relationship between the size and function of the islets. In general, native small islets are superior to large ones in terms of insulin secretion and cell survival both in vivo and in vitro [8, 9, 10, 11]. For instance, native smaller rat islets released a larger amount of insulin in in vitro culture and were highly effective in achieving euglycemia when compared with larger ones when they were transplanted into diabetic rats [10].
Recently, various techniques have been proposed to artificially fabricate 3D cellular aggregates that can mimic the microenvironments of in vivo tissues. One of the most frequently employed strategies is the preparation of multicellular spheroids/aggregates. Microfabricated non-cell-adhesive wells, hanging-drop, and 3-dimentional (3-D) suspension culture techniques have been used to make aggregates of islet cells, hepatocytes, cancer cells, embryonic stem (ES) cells, and induced pluripotent stem (iPS) cells [12, 13]. When cells (e.g., islet cells, β cell line, etc.) are seeded in an environment with non-cell-adhesive surfaces, the cells spontaneously aggregate to form spheroids [14, 15, 16, 17, 18, 19, 20, 21]. Using the dispersed islet cells, attempts have been made to prepare islet cell aggregates (pseudo-islets) that contain β cells with restored insulin secretion activity comparable to that of native islets [14, 15, 16, 18]. In addition, pseudo-islets incorporating adipose-derived stem cells were created to enhance insulin secretion for a long period of time [21]. Microfabricated chambers allow us to form pseudo-islets with precisely controlled sized, which is suitable to investigate the size effect on the islet cell function and viability. For example, Sakai, et al. investigated the effect of O2 tension and size on the function of β cell line (MIN-6 cells) aggregates using oxygen-permeable PDMS chambers [20]. However, the effects of the size of the re-assembled pseudo-islets from primary islet cells on the cell function and morphology have not been investigated in detail. We hypothesized that if the islet cells are re-assembled into pseudo-islets with an optimal size, the function and survival of the islet cells would be enhanced. Such precisely microengineered pseudo-islets would be advantageous when they are transplanted in vivo for treating type 1 diabetics. In this study, we fabricated several types of non-cell-adhesive hydrogel microwells with different diameters to prepare pseudo-islets with well-controlled sizes from dispersed rat islet cells. We examined the size effects of microengineered pseudo-islets on cell viability, distribution of hypoxic cells, arrangement of α/β cells composing the pseudo-islets, and insulin secretion ability of β cells in vitro, to demonstrate the usefulness and the potential applicability of the size-controlled pseudo-islets.
2. Materials and methods
2.1. Isolation of rat islets and preparation of single cells
All animal studies were performed with the approval of the Institutional Animal Care and Use Committee of Tokyo Women’s Medical University. Pancreatic islets were isolated from 9–12 week-old male Lewis rats (Charles River Laboratories, Yokohama, Japan) via collagenase digestion and density-gradient centrifugation using Histopaque solutions with different densities (1.119, 1.100, and 1.077 g/mL, prepared from Histopaque 1119 and 1077, Sigma-Aldrich, St. Louis, MO) as previously described [22]. After isolation, the islets were hand-picked under a stereomicroscope and cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (FBS), 5.5 mmol/L glucose, 10 mmol/L HEPES, 1 mmol/L sodium pyruvate, 0.1 mmol/L nonessential amino acids, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37 °C and 5% CO2 overnight to recover the damage of islets by collagenase treatment [23]. Then, the islets were digested using 0.25% Trypsin-EDTA (Thermo Fisher Scientific) to obtain single cells as previously described [24] and re-suspended in the culture medium at a concentration of 7.5 × 106 cells/mL. The viability of the dispersed islet cells was approximately 90% right after trypsin/EDTA treatment. The percentages of α and β just after islet digestion were 10.7 ± 3.2% and 78.2 ± 4.6%, respectively, which were determined by immunohistochemistry (details are described in "Double-immunohistochemistry for insulin and glucagon" section).
2.2. Fabrication of agarose gel-based microwells
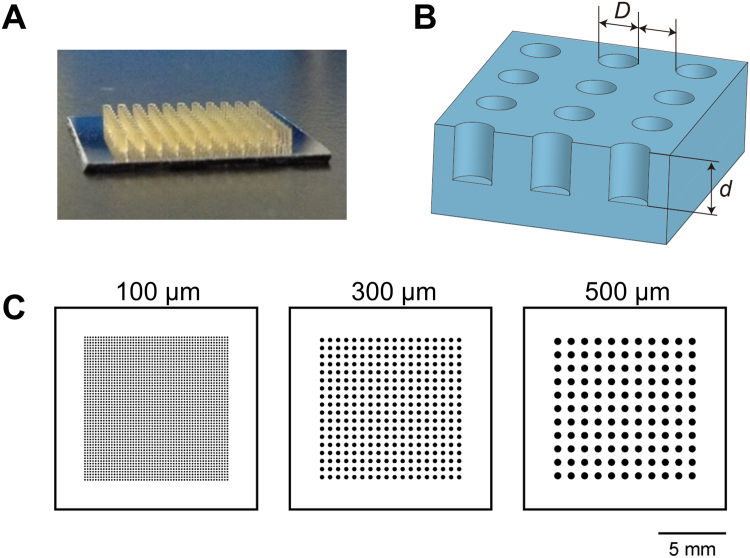
The microwell design is shown in Fig. 1A–C. We employed three different sizes of agarose gel-based microwells with diameters of 100, 300, and 500 μm. The distance between the microwells was equal to the microwell diameter. The depth of the microwell was approximately twice the length of the diameter. The total areas of the microwells were almost the same in the three types of microwell plates. There were 54 × 54, 18 × 18, and 11 × 11 microwells per 100, 300, and 500 μm microwell plate, respectively. The micromolds for the agarose gel plates with microwells were prepared using soft lithography, as described previously [25]. Briefly, cylindrical structures made of a negative photoresist (SU-8 3050, Nippon Kayaku, Tokyo, Japan) were patterned on a silicon wafer with a size of 15 × 15 mm by photolithography and developed using ethyl lactate. The prepared mold was placed in a 6-well plate. Seven mL of an aqueous 3% agarose (Agarose L03, Takara Bio, Shiga, Japan) in saline, dissolved via heating, was poured into the well. After cooling at room temperature for 1 h, the agarose gel plate was removed from the molds, transferred to another 6-well culture plate, and equilibrated with the cell culture medium for 15 min at room temperature. The medium was changed twice within 1 h.
Fig. 1.
The design of agarose gel-based microwells. (A) A micromold to fabricate an agarose gel-based microwell plate. (B) Illustration of an agarose gel-based microwell plate. The distance between the microwells was equal to that of the microwell diameter [D]. The depth [d] of the microwell was approximately twice that of the diameter [D]. (C) Three different sizes of agarose gel-based plates with diameters of 100, 300, and 500 μm microwells. The total areas of the microwells were almost equal in three types of agarose gel-based microwell plates. There were 54 × 54, 18 × 18, and 11 × 11 microwells for 100, 300, 500 μm plates, respectively. Scale bar = 5 mm.
2.3. Formation of size-controlled pseudo-islets
Two hundred μL of the single cell suspension (7.5 × 106 cells/mL) was pipetted onto the agarose gel plate with the microwells. The number of the seeded cells was optimized so that microwells were filled with islet cells and the numbers of the introduced cells for all the wells became constant. After cell seeding, the culture plate was shaken gently for 5 min on a plate shaker to distribute the cells evenly into the microwells, and then it was incubated at room temperature for 10 min. Then, 2 mL of the culture medium was gently added to the culture plate. The cells in the microwells were cultured at 37 °C in 5% CO2 for 7 days. As a control, isolated intact islets were also cultured on an agarose gel plate without microwells.
2.4. Size measurement of microengineered pseudo-islets
Micrographs of islet cell aggregations were taken in 3–5 randomly selected fields per a well using an inverted microscope at 7 days after cultivation. The size of the aggregates was calculated from digital images using Image J software (National Institutes of Health, Bethesda, MD) (n = 30 ∼ 105).
2.5. Assessment of cell viability in microengineered pseudo-islets
The cell survival and death of pseudo-islets were assessed by the LIVE/DEAD Viability/Cytotoxicity Kit (Thermo Fisher Scientific) at 7 days after cultivation. The aggregates were incubated in a mixture of 4 μM calcein AM (live cell, green) and 8 μM ethidium homodimer-1 (dead cell, red) for 45 min at room temperature. The confocal image stacks of aggregates were acquired by a confocal laser-scanning microscope (FLUOVIEW FV1200; Olympus, Tokyo, Japan). The ratio of the red-colored area to the total cell area (%) was calculated from the confocal images of the aggregates in 3–4 randomly selected fields per sample using Image J software.
2.6. Detection of hypoxic cells
Hypoxic cells in the pseudo-islets were detected by a Hypoxyprobe-1 Kit (Hypoxyprobe, Burlington, MA). After 7 days of cultivation, the aggregates in the microwells were incubated for 2 h in the presence of 200 μM pimonidazole hydrochloride (Hypoxyprobe-1). Then, the aggregates were collected, embedded in iPGell (GenoStaff, Tokyo, Japan), and fixed with 4% paraformaldehyde (PFA) for 4 h according to the manufacturer's instructions. The gels containing aggregates were embedded in paraffin and sectioned at 5 μm. The sections were incubated with mouse monoclonal anti-pimonidazole antibody overnight at 4 °C. Alexa Fluor 488-conjugated goat anti-mouse IgG antibody (Thermo Fisher Scientific) was used as the secondary antibody. The sections were mounted with ProLong Gold Antifade Reagent with DAPI (Thermo Fisher Scientific). The images were taken with a fluorescence microscope (Olympus BX51).
2.7. Double-immunohistochemistry for insulin and glucagon
After 7 days of cultivation, pseudo-islets were collected from microwells. Pseudo-islets and intact islets (cultured for 0 or 7 days) were embedded in iPGell, and fixed with 4% PFA. The fixed cells were embedded in paraffin and sectioned at 5 μm. The sections were incubated with guinea-pig polyclonal anti-insulin antibody (Abcam, Cambridge, UK) and with mouse monoclonal anti-glucagon antibody (Abcam) overnight at 4 °C. For double-immunofluorescent staining, the sections were treated with both Alexa Fluor 488-conjugated goat anti-guinea pig IgG and Alexa Fluor 594-conjugated goat anti-mouse IgG (Thermo Fisher Scientific) for 30 min. The images were obtained with a fluorescence microscope. The numbers of β cells (insulin-positive cells), α cells (glucagon-positive cells), and the total cells (DAPI) were counted on the images obtained by immunohistochemistry for each condition, and the percentage of each cell type was calculated.
2.8. Glucose-induced insulin secretion assay
After 7 days of cultivation, pseudo-islets were preincubated for 3 h at 37 °C with the cell culture medium containing 3.3 mmol/L glucose. Subsequently, the cells were incubated with fresh medium containing 3.3 mmol/L glucose for 1 h. Then, the medium was replaced with medium containing 20 mmol/L glucose, and the cells were incubated for 1 h. At the end of each step, the medium was collected for determination of the insulin level. The concentration of insulin in the medium was measured by the Mercodia Rat Insulin ELISA Kit (Mercodia, Uppsala, Sweden) according to the manufacturer's instructions. The relative cell viability of the islet cells was determined by a WST-8 (water-soluble tetrazolium salt) assay using Cell Count Reagent SF kit (Nacalai Tesque, Kyoto, Japan). In addition, the DNA content of the pseudo-islets was determined by a DNA quantitation kit (Bio-Rad, Hercules, CA). The values of insulin secretion were normalized to the absorbance values from WST-8 assay or the DNA content in the islet cells.
2.9. Statistical analysis
All data are expressed as the mean ± standard deviation (SD). Significant differences between the groups were tested using analysis of variance (ANOVA) followed by a Tukey honestly significant difference (HSD) post-hoc test with IBM SPSS Statistics 18 software (IBM Japan, Tokyo). A probability value less than 0.05 (P < 0.05) was considered to be statistically significant.
3. Results
3.1. Formation of size-controlled pseudo-islets using rat islet cells
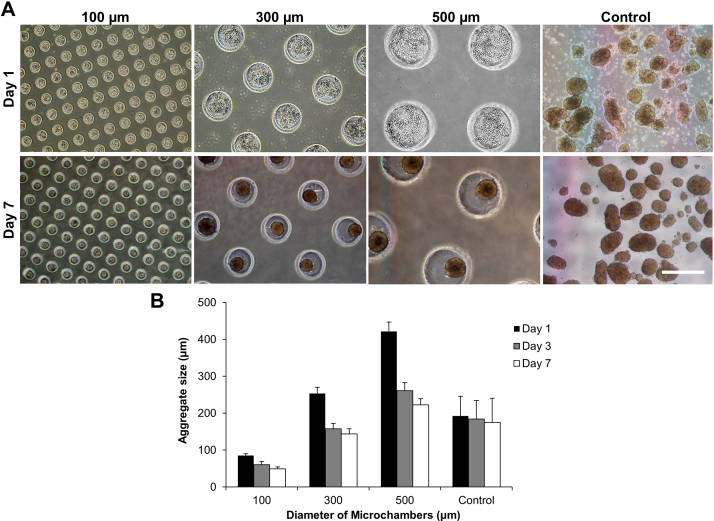
Islets were freshly isolated from Lewis rats, and then dispersed with trypsin/EDTA into a suspension of single islet cells. To form aggregates of rat islet cells, a suspension of the dispersed islet cells at a concentration of 7.5 × 106 cells/mL was pipetted onto the agarose gel plates containing microwells of different sized diameters (100, 300, or 500 μm). The numbers of the islet cells introduced into the 100, 300, and 500 μm microwells were estimated to be approximately 500, 4,600, and 12,400 cells for each microwell, respectively. During cell cultivation, the single islet cells spontaneously assembled into aggregates because of the non-cell-adhesive nature of the agarose hydrogel, and islet-like round aggregates (pseudo-islets) formed after several days of cultivation. The aggregate sizes in the microwells varied depending on the well diameter, mainly because of the different amounts of the inoculated cells per microwell. Fig. 2A shows the pseudo-islets formed in the microwells at 1 and 7 days after cultivation. After 1 day of cultivation, cells were loosely aggregated in the microwells. Fig. 2B shows the time-course change of the pseudo-islet size in the microwells. Islet-like spherical aggregates spontaneously formed in the microwells within 3 days. The size of the pseudo-islets was smaller than that of the microwells, due to contraction among the cells. After 7 days of cultivation, the average diameters ± SD of the pseudo-islets in 100, 300, and 500 μm microwells were 49.5 ± 4.9 μm, 144.1 ± 13.7 μm, 222.8 ± 16.6 μm, respectively, while the intact control islets were 174.9 ± 65.8 μm (Fig. 2B). The sizes of the intact islets were not significantly changed during cultivation for 7 days, but the size distribution was larger. These results clearly demonstrated the ability of the microfabricated microwell-based cultivation technique for precisely controlling the size of the assembled 3D islet tissue-like constructs.
Fig. 2.
The formation of size-controlled pseudo-islets using agarose gel-based microwells. (A) Dispersed rat islet cells were cultured in 100 μm, 300 μm, and 500 μm agarose-based microwells for 1 and 7 days. Intact islets were also cultured on an agarose gel plate without microwells as control. The aggregate size increased with increasing diameter of the agarose-based microwell. Scale bar = 500 μm. (B) The diameter of the pseudo-islets prepared using 100, 300, and 500 μm microwells and cultured intact islets at 1, 3, and 7 days after cultivation. Data are presented as the mean ± SD (n = 30 ∼ 105).
3.2. Detection of both live/dead cells and hypoxic cells in the microengineered pseudo-isles
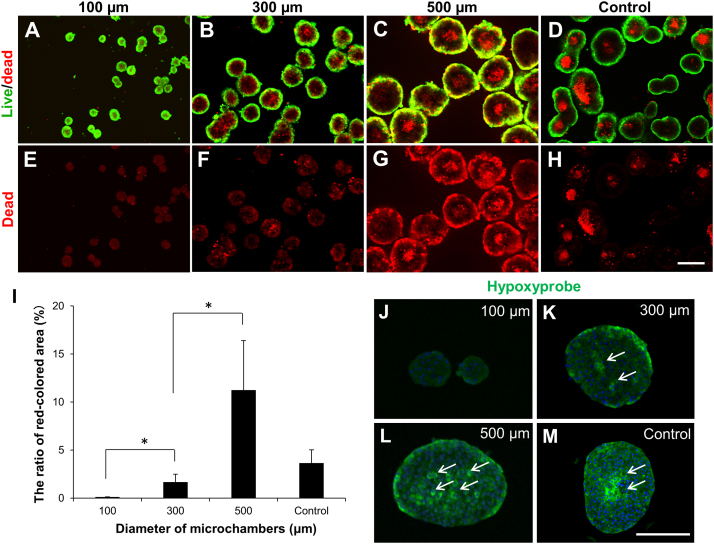
To determine the live and dead cells in the formed pseudo-islets and the intact islets, the aggregates comprising the islet cells were stained with calcein AM (live, green) and ethidium homodimer-1 (EthD-1; dead, red) (Fig. 3A–D) or EthD-1 alone (Fig. 3E–H) at day 7. In the relatively large pseudo-islets prepared using 300 μm and 500 μm microwells (Fig. 3B, C, F, G) and the large-sized intact islets (Fig. 3D, H), live cells localized at the periphery while cell death occurred mainly in the central core region. In contrast, dead cells were rarely observed in the small pseudo-islets prepared using the 100 μm microwells (Fig. 3A, E) and in the small-sized intact islets (Fig. 3D, H). The ratios of red-stained area to total area (%), which are related to the ratios of dead cells, in the pseudo-islets prepared using 100, 300 and 500 μm microwells and in the intact islets were 0.11 ± 0.02%, 1.68 ± 0.82%, 11.26 ± 5.13%, and 3.66 ± 1.38%, respectively, indicating that the small size resulted in significantly less cell death (Fig. 3I). We then determined the presence of hypoxic cells in the pseudo-islets and intact islets using the Hypoxyprobe-1 Kit at day 7 (Fig. 3J–M). As shown in Fig. 3J, the pseudo-islets prepared using the 100 μm microwells had little or no hypoxic cells in the central core region. However, the number of hypoxic cells increased in the core with the increase in the aggregate size (Fig. 3 K, L), suggesting that the hypoxic condition induced islet cell death in the pseudo-islets larger than 150 μm. In the pseudo-islets prepared using 500 μm microwells, hypoxic cells were partially present in the peripheral region, which possibly indicates that cells locating near the bottom of the microwell were in a hypoxic condition because of the limited oxygen supply. In the relatively large-sized intact islets, hypoxic cells were observed near the center (Fig. 3 M).
Fig. 3.
Detection of cell death and hypoxic cells in size-controlled pseudo-islets. (A–H) Confocal images of (A–D) live/dead staining and (E–H) dead staining alone of pseudo-islets prepared using (A, E) 100 μm, (B, F) 300 μm, and (C, G) 500 μm microwells and (D, H) intact islets (control) at day 7. Aggregates were stained with calcein AM (live cell, green) and with ethidium homodimer-1 (dead cell, red). (I) The level of cell death in the pseudo-islets prepared using 100, 300, and 500 μm microwells and intact islets (control). The ratio of red stained area to total area of aggregates (%) was calculated from 3–4 randomly selected fields/sample using Image J software. Data are presented as the mean ± SD. *Significant differences between groups (p < 0.05). (J–M) Immunostaining for hypoxyprobe (pimonidazole) in cross-sections of pseudo-islets prepared using (J) 100 μm, (K) 300 μm, and (L) 500 μm microwells and (M) intact islets (control). Aggregates were incubated in the presence of 200 μM pimonidazole hydrochloride to detect hypoxic cells in aggregates at 7 days after cultivation. Arrows shows hypoxyprobe-positive cells. Scale bars = 200 μm.
3.3. Localization of α cells and β cells in the microengineered pseudo-islets
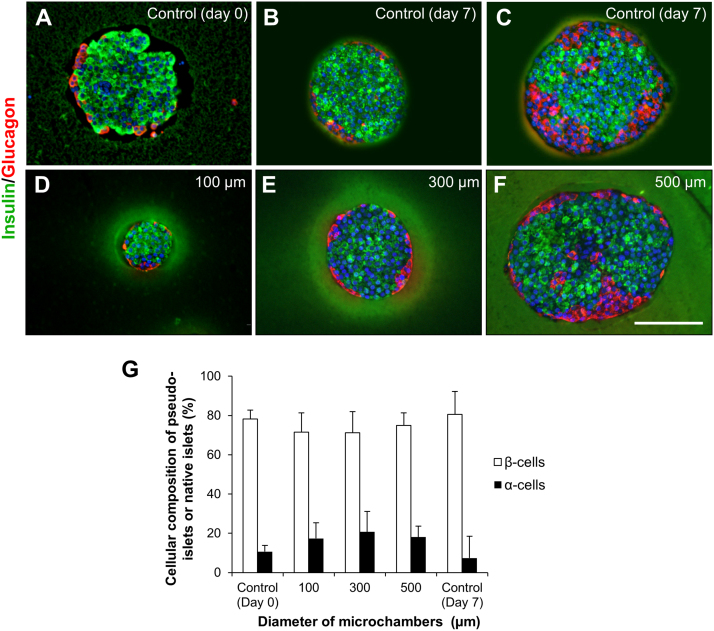
In rodent islets, the insulin producing β cells localize in the central area of islets, while the glucagon producing α cells localize at the periphery, surrounding the β cells [26], as shown in Fig. 4A. We investigated the arrangement of the α and β cells in the reconstituted pseudo-islets by immunohistochemistry (Fig. 4). After 7 days of cultivation, the insulin- and glucagon-positive cells were observed in the pseudo-islets prepared using 100, 300, and 500 μm microwells (Fig. 4D–F). In the pseudo-islets prepared using 100 and 300 μm microwells, insulin- and glucagon-positive cells were distributed in the central area and in the periphery, respectively (Fig. 4D, E). This characteristic cellular arrangement is morphologically similar to that observed in the relatively small native islets at day 0 and 7 (Fig. 4A, B). In contrast, several glucagon-positive cells were located in the core region of the large pseudo-islets prepared using the 500 μm microwells, as in the case of the large-sized intact islets at day 7 (Fig. 4C, F). These data suggest that adequate reconstitution of islet-like arrangement of islet cells was induced in both small and medium pseudo-islets prepared using 100 and 300 μm microwells. Fig. 4G shows the ratio of α and β cells composing the pseudo-islets and native islets. There was no significant difference in the cellular compositions of α and β cells between pseudo-islets prepared using three types of microwells at day 7, while the ratios of α cells in pseudo-islets were slightly higher compared to that of the intact islets at day 0.
Fig. 4.
Cell arrangement and composition of microengineered pseudo-islets and intact islets. (A–F) Immunohistochemisty for insulin (green) and glucagon (red) in cross-sections of intact islets at (A) day 0 and (B, C) day 7 (control) and pseudo-islets prepared using (D) 100 μm, (E) 300 μm, and (F) 500 μm microwells at 7 days after cultivation. Scale bar = 200 μm. (G) The ratio of α and β cells in the intact islets prior to dissociation (control) at day 0, pseudo-islets prepared using 100 μm, 300 μm, and 500 μm microwells at day 7, and cultured intact islets (control) at day 7. The numbers of β cells (insulin positive cells), α cells (glucagon positive cells), and total cells (DAPI) were counted on the images obtained by immunohistochemistry (n = 18 ∼ 22).
3.4. Insulin secretory capacity of size-controlled pseudo-islets
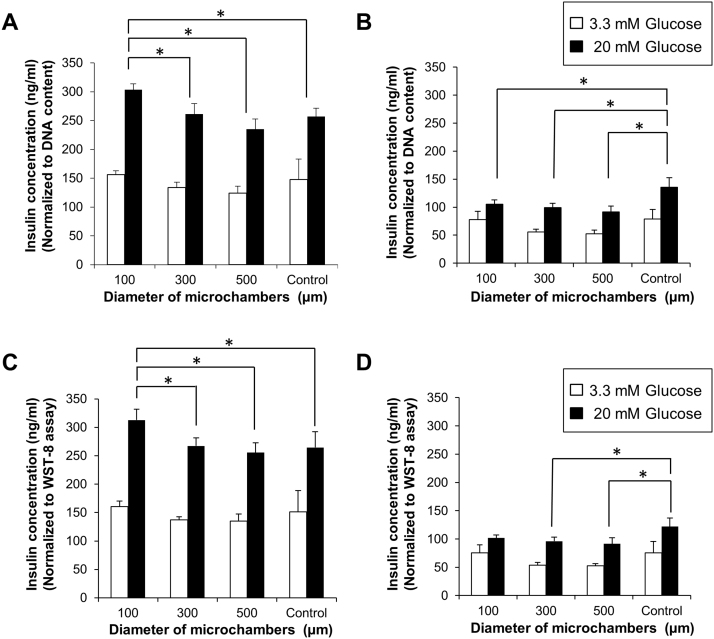
We next sought to assess the functional capacity of the pseudo-islets. After 7 and 14 days of cultivation, pseudo-islets prepared using 100, 300, and 500 μm microwells and cultured intact control islets were exposed to media containing basal glucose (3.3 mM), and then the cultures were exposed to media containing high glucose (20 mM). The concentrations of insulin secreted into the culture media with basal/high glucose levels normalized to DNA content are shown in Fig. 5A, B. In addition, the insulin secretions normalized to the cell metabolic activity based on WST-8 assay are shown in Fig. 5C, D. At day 7, insulin secretion was stimulated by exposure to a high glucose medium, showing that the insulin secretory responsiveness of the islet cells composing the pseudo-islets was preserved (Fig. 5A, C). Notably, the insulin release from the pseudo-islets prepared using the 100 μm microwells at the high glucose level was significantly higher than that secreted by the pseudo-islets prepared in the 300 and 500 μm microwells and intact islets. This result indicates that small pseudo-islets had a much greater ability to secrete insulin in response to glucose stimulation, likely due to the high surface-to-volume ratio and the short diffusion distance for efficiently supplying oxygen and nutrition. At day 14, the insulin secretion levels of the pseudo-islets were decreased in all conditions using different-sized microwells (Fig. 5B, D). There was no statistical difference between groups of pseudo-islets. The insulin secretion level of the intact islets was also decreased compared to that at day 7, but its decrease rate was slightly suppressed compared to those of pseudo-islets. This result indicates that the remaining ECM components in the intact islets might have contributed to the maintenance of β cell function and islet cell survival, as described in a previous study [27]. Further optimization of the cultured conditions will be possible, such as incorporation of ECM components [25] into the pseudo-islets.
Fig. 5.
Glucose stimulated insulin secretion from pseudo-islets and intact islets. (A–D) Pseudo-islets formed in 100 μm, 300 μm, and 500 μm microwells (n = 6) and cultured intact islets (control; n = 6) were incubated with 3.3 mmol/L glucose and subsequently 20 mmol/L glucose at (A, C) day 7 and (B, D) day 14. Insulin concentration was normalized to (A, B) the DNA content in the islet cells or (C, D) the absorbance values from WST-8 assay. Data are presented as the mean ± SD. *Significant differences between groups (p < 0.05).
4. Discussion
Sufficient oxygen and nutrients are supplied by the blood circulation to pancreatic islets in vivo, which is indispensable to both the survival and hormone secretion of islet cells. It was previously reported that in the presence of high glucose insulin secretion by mouse insulinoma βTC3 cells at a partial oxygen tension (pO2) less than 7 mmHg was significantly lower than that at a higher pO2 [28]. In addition, the pO2 is decreased with distance from a blood vessel in in vivo tissues. The pO2 level was less than 10 mmHg at a distance of 50 μm from a vessel wall in the arterioles in the rat mesentery [29]. In the case of islets isolated from the body via collagenase perfusion, the microcirculation through the islet tissue is no longer present. Therefore, oxygen and nutrients are supplied to the isolated/transplanted islets only by diffusion from the culture medium and from the surrounding tissues, respectively. The absence of a circulation within islets leads to an insufficient supply of oxygen and nutrients, especially for cells in the core region of large islets (> 100 μm). In fact, the pO2 level in the transplanted islet grafts was extremely low (∼5 mmHg) compared with that in the in vivo islets (∼40 mmHg) regardless of the transplantation site (kidney, liver, and spleen) [30], because of the insufficient revascularization within the islet grafts. It is therefore logical that the superiority of small islets is primarily due to the short diffusion distance. Researchers demonstrated that native small islets isolated from the pancreas are superior to large ones when either cultured in vitro or transplanted in vivo [8, 9, 10, 11]. The superiority of the native small islets has also been studied at the molecular level. A previous study reported that the expression of angiotensinogen was significantly low but that of VEGF-A was high in small islet grafts, indicating the improved microcirculation and enhanced revascularization in the transplanted small islets, respectively [9].
Selection of native small islets from isolated islets for transplantation therapy of type 1 diabetes is not practical, due to the worldwide shortage of pancreas/islet donors. The islet cells dispersed by trypsinization have an ability to reconstitute aggregates, and the reorganized pseudo-islets retain the ability to secrete insulin at levels comparable to that of native islets [14]. Hence, we hypothesized that a better therapeutic approach for transplantation can be realized if islets of various sizes are dispersed as single cells and then reorganized into small islet-like aggregates with both a higher function and longer survival. However, conventional culture methods were not able to precisely control the size of the pseudo-islets.
Recently, tissue engineering techniques have been newly developed to fabricate functional micro-tissues using cells and biochemical materials. Islet cell sheets for transplantation were created using thermo-responsive culture dishes coated with laminin-5 [24, 31]. Engineered pseudo-islets have also been prepared using either precisely fabricated agarose-gel microwells [32, 33] or alginate hydrogel beads [34]. However, to date, preparation of aggregates of islet cells with a well-controlled size and their characterization have not been accomplished. In this study, we employed precisely fabricated agarose hydrogel microwell platforms to prepare size-controlled pseudo-islets from rat islet cells. Agarose microwells with different diameters enabled us to create reliable aggregates of different sizes, when the amounts of inoculated cells were controlled.
Another important finding in the present study is that a physiological cellular organization of the α and β cells occurred in both the small and medium pseudo-islets prepared using 100 and 300 μm microwells, respectively. This morphology was less reconstituted in the large-sized pseudo-islets prepared using 500 μm microwells. In vivo-like organization of the islet cells (α and β cells) in pseudo-islets has been previously reported both in vitro [14, 15, 16] and in vivo [35, 36], but the in vivo-like cellular arrangement of islet cells in 3D culture platforms with precisely controlled sizes has not been reported, and the size effect on the micro-tissue organization was not fully investigated. Our findings clearly showed that the cellular organization of both the α and β cells is closely associated with the pseudo-islet size, suggesting that the aggregation process also requires a supply of oxygen and nutrients in the pseudo-islets.
Recently, some research groups have succeeded in achieving reversal of hyperglycemia by transplanting ES or iPS cell-derived β-like cells in diabetic mice [37, 38, 39]. In these studies, the unprocessed stem cell-derived β-like cells were used for transplantation. We expect that transplantation of 3D engineered islet-like tissues with an appropriate size and composed of stem cell-derived multiple types of cells (α and β cells) may have the potential to provide a more efficient therapeutic approach for treating type 1 diabetes when compared to that with transplantation of dispersed cells.
In summary, the present study demonstrated the close relationship between the size and various cellular characteristics, including cell viability and functions, in the microengineered pseudo-islets. The results clearly showed that the reconstituted small pseudo-islets (45–55 μm) have a higher insulin secretion ability and significantly less cell death than those of the medium (130–160 μm) and large (200–240 μm) ones. In vivo-like cellular organization was promoted in both the small and medium pseudo-islets. The presented approach for re-organizing islet cells into the pseudo-islets with appropriate sizes using microfabricated well structures will be highly useful and effective not only for transplantation therapy but also for studying the cellular physiology of islets cells in in vivo tissue-like microenvironments.
Declarations
Author contribution statement
Yumie Ichihara: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Rie Utoh: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Masumi Yamada: Performed the experiments; Contributed reagents, materials, analysis tools or data; Analyzed and interpreted the data; Wrote the paper.
Tatsuya Shimizu, Yasuko Uchigata: Analyzed and interpreted the data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported in part by the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems (T.S.) from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, a grant from the Pancreas Research Foundation of Japan (Y.I.), and the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 26350530 (R.U.), 16J40041 (R.U.), and 23106007 (M.Y.).
Additional information
No additional information is available for this paper.
Acknowledgments
We thank Dr. Masafumi Goto (Tohoku University) for providing a technique for rat islet isolation.
References
- 1.Shapiro A.M.J., Lakey J.R.T., Ryan E.A., Korbutt G.S., Toth E., Warnock G.L., Kneteman N.M., Rajotte R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Posselt A.M., Bellin M.D., Tavakol M., Szot G.L., Frassetto L.A., Masharani U., Kerlan R.K., Fong L., Vincenti F.G., Hering B.J., Bluestone A., Stock P.G. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am. J. Transplant. 2010;10:1870–1880. doi: 10.1111/j.1600-6143.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Adra D.P., Gill R.S., Imes S., O'Gorman D., Kin T., Axford S.J., Shi X., Senior P.A., Shapiro A.M. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation. 2014;98:1007–1012. doi: 10.1097/TP.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 4.McCall M., Shapiro A.M. Update on islet transplantation. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan E.A., Paty B.W., Senior P.A., Bigam D., Alfadhli E., Kneteman N.M., Lakey J.R., Shapiro A.M. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro A.M. Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev. Diabet. Stud. 2012;9:385–406. doi: 10.1900/RDS.2012.9.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elayat A.A., el-Naggar M.M., Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J. Anat. 1995;186(Pt 3):629–637. [PMC free article] [PubMed] [Google Scholar]
- 8.Lehmann R., Zuellig R.A., Kugelmeier P., Baenninger P.B., Moritz W., Perren A., Clavien P.A., Weber M., Spinas G.A. Superiority of small islets in human islet transplantation. Diabetes. 2007;56:594–603. doi: 10.2337/db06-0779. [DOI] [PubMed] [Google Scholar]
- 9.Li W., Zhao R., Liu J., Tian M., Lu Y., He T., Cheng M., Liang K., Li X., Wang X., Sun Y., Chen L. Small islets transplantation superiority to large ones: implications from islet microcirculation and revascularization. J. Diabetes Res. 2014;2014:192093. doi: 10.1155/2014/192093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacGregor R.R., Williams S.J., Tong P.Y., Kover K., Moore W.V., Stehno-Bittel L. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am. J. Physiol. Endocrinol. Metab. 2006;290:E771–E779. doi: 10.1152/ajpendo.00097.2005. [DOI] [PubMed] [Google Scholar]
- 11.Vakhshiteh F., Allaudin Z.N., Mohd Lila M.A., Hani H. Size-related assessment on viability and insulin secretion of caprine islets in vitro. Xenotransplantation. 2013;20:82–88. doi: 10.1111/xen.12023. [DOI] [PubMed] [Google Scholar]
- 12.Fennema E., Rivron N., Rouwkema J., van Blitterswijk C., de Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013;31:108–115. doi: 10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Cavallari G., Zuellig R.A., Lehmann R., Weber M., Moritz W. Rat pancreatic islet size standardization by the hanging drop technique. Transplant. Proc. 2007;39:2018–2020. doi: 10.1016/j.transproceed.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Halban P.A., Powers S.L., George K.L., Bonner-Weir S. Spontaneous reassociation of dispersed adult rat pancreatic islet cells into aggregates with three-dimensional architecture typical of native islets. Diabetes. 1987;36:783–790. doi: 10.2337/diab.36.7.783. [DOI] [PubMed] [Google Scholar]
- 15.Hopcroft D.W., Mason D.R., Scott R.S. Insulin secretion from perifused rat pancreatic pseudoislets. In Vitro Cell Dev. Biol. 1985;21:421–427. doi: 10.1007/BF02620828. [DOI] [PubMed] [Google Scholar]
- 16.Matta S.G., Wobken J.D., Williams F.G., Bauer G.E. Pancreatic islet cell reaggregation systems: efficiency of cell reassociation and endocrine cell topography of rat islet-like aggregates. Pancreas. 1994;9:439–449. [PubMed] [Google Scholar]
- 17.O'Sullivan E.S., Johnson A.S., Omer A., Hollister-Lock J., Bonner-Weir S., Colton C.K., Weir G.C. Rat islet cell aggregates are superior to islets for transplantation in microcapsules. Diabetologia. 2010;53:937–945. doi: 10.1007/s00125-009-1653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang J.W., Lee B.R., Jung M.J., Jung H.S., Hwang Y.H., Kim M.J., Lee S.H., Lee D.Y. Functional clustering of pancreatic islet cells using concave microwell array. Macromol. Res. 2011;19:1320–1326. [Google Scholar]
- 19.Bernard A.B., Lin C.C., Anseth K.S. A microwell cell culture platform for the aggregation of pancreatic β-cells. Tissue Eng. Part C Methods. 2012;18:583–592. doi: 10.1089/ten.tec.2011.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinohara M., Kimura H., Montagne K., Komori K., Fujii T., Sakai Y. Combination of microwell structures and direct oxygenation enables efficient and size-regulated aggregate formation of an insulin-secreting pancreatic β-cell line. Biotechnol. Prog. 2014;30:178–187. doi: 10.1002/btpr.1837. [DOI] [PubMed] [Google Scholar]
- 21.Jun Y., Kang A.R., Lee J.S., Park S.J., Lee D.Y., Moon S.H., Lee S.H. Microchip-based engineering of super-pancreatic islets supported by adipose-derived stem cells. Biomaterials. 2014;35:4815–4826. doi: 10.1016/j.biomaterials.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 22.Saito Y., Goto M., Maya K., Ogawa N., Fujimori K., Kurokawa Y., Satomi S. Brain death in combination with warm ischemic stress during isolation procedures induces the expression of crucial inflammatory mediators in the isolated islets. Cell Transplant. 2010;19:775–782. doi: 10.3727/096368910X508889. [DOI] [PubMed] [Google Scholar]
- 23.Carter J.D., Dula S.B., Corbin K.L., Wu R., Nunemaker C.S. A practical guide to rodent islet isolation and assessment. Biol. Proced. Online. 2009;11:3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu H., Ohashi K., Utoh R., Ise K., Gotoh M., Yamato M., Okano T. Bioengineering of a functional sheet of islet cells for the treatment of diabetes mellitus. Biomaterials. 2009;30:5943–5949. doi: 10.1016/j.biomaterials.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Yamada M., Hori A., Sugaya S., Yajima Y., Utoh R., Yamato M., Seki M. Cell-sized condensed collagen microparticles for preparing microengineered composite spheroids of primary hepatocytes. Lab Chip. 2015;15:3941–3951. doi: 10.1039/c5lc00785b. [DOI] [PubMed] [Google Scholar]
- 26.Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982;31:538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- 27.Nagata N., Gu Y., Hori H., Balamurugan A.N., Touma M., Kawakami Y., Wang W., Baba T.T., Satake A., Nozawa M., Tabata Y., Inoue K. Evaluation of insulin secretion of isolated rat islets cultured in extracellular matrix. Cell Transplant. 2001;10:447–451. [PubMed] [Google Scholar]
- 28.Papas K.K., Long R.C., Jr., Constantinidis I., Sambanis A. Effects of oxygen on metabolic and secretory activities of βTC3 cells. Biochim. Biophys. Acta. 1996;1291:163–166. doi: 10.1016/0304-4165(96)00062-1. [DOI] [PubMed] [Google Scholar]
- 29.Tsai A.G., Friesenecker B., Mazzoni M.C., Kerger H., Buerk D.G., Johnson P.C., Intaglietta M. Microvascular and tissue oxygen gradients in the rat mesentery. Proc. Natl. Acad. Sci. U S A. 1998;95:6590–6595. doi: 10.1073/pnas.95.12.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsson P.O., Palm F., Andersson A., Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489–495. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 31.Saito T., Ohashi K., Utoh R., Shimizu H., Ise K., Suzuki H., Yamato M., Okano T., Gotoh M. Reversal of diabetes by the creation of neo-islet tissues into a subcutaneous site using islet cell sheets. Transplantation. 2011;92:1231–1236. doi: 10.1097/TP.0b013e3182375835. [DOI] [PubMed] [Google Scholar]
- 32.Hoffecker I.T., Iwata H. Manipulation of cell sorting within mesenchymal stromal cell-islet cell multicellular spheroids. Tissue Eng. Part A. 2014;20:1643–1653. doi: 10.1089/ten.TEA.2013.0305. [DOI] [PubMed] [Google Scholar]
- 33.Hilderink J., Spijker S., Carlotti F., Lange L., Engelse M., van Blitterswijk C., de Koning E., Karperien M., van Apeldoorn A. Controlled aggregation of primary human pancreatic islet cells leads to glucose-responsive pseudoislets comparable to native islets. J. Cell Mol. Med. 2015;19:1836–1846. doi: 10.1111/jcmm.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kojima N. In vitro reconstitution of pancreatic islets. Organogenesis. 2014;10:225–230. doi: 10.4161/org.28351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu H., Ohashi K., Saito T., Utoh R., Ise K., Yamato M., Okano T., Gotoh M. Topographical arrangement of α- and β-cells within neo-islet tissues engineered by islet cell sheet transplantation in mice. Transplant. Proc. 2013;45:1881–1884. doi: 10.1016/j.transproceed.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Lavallard V., Armanet M., Parnaud G., Meyer J., Barbieux C., Montanari E., Meier R., Morel P., Berney T., Bosco D. Cell rearrangement in transplanted human islets. FASEB J. 2015 doi: 10.1096/fj.15-273805. [DOI] [PubMed] [Google Scholar]
- 37.Alipio Z., Liao W., Roemer E.J., Waner M., Fink L.M., Ward D.C., Ma Y. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic β-like cells. Proc. Natl. Acad. Sci. U S A. 2010;107:13426–13431. doi: 10.1073/pnas.1007884107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A., O'Dwyer S., Quiskamp N., Mojibian M., Albrecht T., Yang Y.H., Johnson J.D., Kieffer T.J. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]