Abstract
Rhodococcus equi is an unusual cause of infection in humans, but has emerged as an opportunistic pathogen among immunocompromised patients. Primary pulmonary involvement is the most common clinical presentation, although the spectrum of disease is broad. Diagnosing R. equi infections remains challenging, both from clinical and microbiological view, and no standard treatment has been established. In this report, we present a detailed case of a 57-year-old male renal transplant recipient who developed R. equi bacteremia with a concomitant Pneumocystis jirovecii pneumonia. We describe the clinical features of R. equi infections, highlight the importance of an early diagnosis, and briefly review treatment options for this rare infection.
Rhodococcus equi is well described in veterinary medicine. Although still not commonplace, it has increasingly been reported as an opportunistic pathogen, particularly in immunocompromised patients, such as solid organ transplant patients. We describe a case of R. equi sepsis in a kidney transplant recipient.
CASE DESCRIPTION
A 57-year-old man presented with cough, hemoptysis, and right-sided pleuritic chest pain accompanied by dyspnea on exertion and general malaise. The patient underwent cadaveric kidney transplantation 7 months before presentation because of end-stage renal disease after having been on chronic hemodialysis treatment for 7 months. The origin of renal insufficiency was not determined, but there was a family history of proteinuria in his mother, twin brother, and both his daughters. The twin brother had undergone a renal transplantation as well. Additional medical history included hypertension, hypercholesterolemia, and asthma bronchiale. Because of signs of borderline acute rejection on the protocol biopsy of the transplanted kidney 4 months after transplantation, corticosteroids were not withdrawn as per protocol. Pneumocystis jirovecii prophylaxis which consisted of trimethoprim-sulfamethoxazole (160-800 mg daily) was stopped after this protocol biopsy in conformity with our standard practice and as the patient was administered only a low dose of corticosteroids.
His maintenance immunosuppressive therapy at the time of presentation consisted of mycophenolate mofetil (Cellcept; Roche, Basel, Switzerland; 500 mg twice per day), tacrolimus (Advagraf; Astellas Pharma Europe, Staines, UK; 17 mg once daily; trough level, 8-12 ng/mL) and methylprednisolone (Medrol; Pfizer, New York; 4 mg once daily). The patient denied any direct exposure to livestock. On physical examination, the patient was in no apparent distress and was afebrile. Laboratory data included a total leukocyte count of 9.11 × 109/L with a differential of 82% segments, 6.6% monocytes, and 10.4% lymphocytes, hemoglobin of 10.6 g/dL (N 14-18), and slightly elevated C-reactive protein (CRP) level of 18.5 mg/L (N < 5.0). Lactate dehydrogenase (LDH) was elevated as well: 378 U/L (N < 248). The patient’s renal allograft function had deteriorated, and he had an estimated glomerular filtration rate of 21 mL/min (previously 26 mL/min after transplantation). Transaminases, bilirubin concentrations, and electrolyte panel were normal. Chest radiographs and computed tomography (CT) obtained during the outpatient workup disclosed a roundish lung lesion measuring 32 × 32 × 32 mm in the left lower lobe, displaying 2 air bronchograms within it and an infracarinal lymphadenopathy (Figure 1A, B). A bronchoscopy with bronchoalveolar lavage (BAL) was performed, and afterward empiric therapy with amoxicillin-clavulanate (2 g twice per day orally) was initiated. At re-evaluation a few days later, there was a favorable clinical evolution, but without resolution of symptoms. The BAL fluid showed a neutrophilic cellular pattern, but all aerobic, anaerobic, mycobacterial, yeast, and fungal cultures, as well as antigen tests for influenza A, B, respiratory syncytial virus, and adenovirus returned as negative. Immunofluorescent assay for P. jirovecii in BAL fluid was negative as well. However, galactomannan test in BAL fluid was positive (3.7, index < 0.5). In view of a positive host and clinical factors, a diagnosis of probable invasive aspergillosis was made. After a new bronchoscopy with peripheral biopsies, therapy with voriconazole (200 mg twice per day) was associated with a concomitant reduction in mycophenolate mofetil dose to 250 mg twice per day and in tacrolimus dose to 8 mg daily. The latter to avoid tacrolimus overexposure caused by drug-drug interaction with voriconazole.
FIGURE 1.
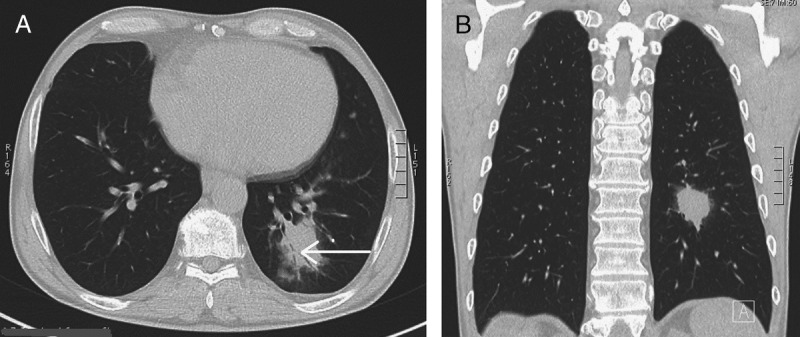
A, B, CT shows an irregular opacity in the left lower lobe. This opacity contains an airbronchogram (arrow) and is surrounded by a ground-glass halo.
A week after initial presentation, the patient was admitted to the emergency department because of low blood pressure (95/40 mm Hg), sudden dizziness, nausea, and atypical chest pain. He was afebrile. Physical examination was unremarkable. His laboratory evaluations revealed worsened renal allograft function (an estimated glomerular filtration rate of 15 mL/min) and further elevated CRP level (35.4 mg/L). Blood cultures were obtained upon admission. Repeat chest radiographs showed the known lung lesion, which seemed unchanged in comparison with previous imaging. Abdominal ultrasound was normal. Electrocardiogram on admission revealed ST segment depression in the inferolateral leads, which in combination with increasing troponin I levels (maximal, 2.88 μg/L; N < 0.10) raised the suspicion of a non-ST elevation myocardial infarction. A reduced dose of low molecular weight heparin was prescribed taking into account the recent history of hemoptysis. The patient was transferred to the cardiac intensive care unit for monitoring. After a few days, blood cultures from admission became positive for R. equi (Figures 2 and 3). Cultures of the second BAL fluid returned all negative again. The antibiotic regimen was changed to intravenous (IV) meropenem (1 g, 3 times per day) and continuous vancomycin infusion (aiming at a plateau plasma concentration of 25 μg/mL) to treat this R. equi sepsis. Immunosuppression was further tapered: mycophenolate mofetil was stopped, and tacrolimus through levels were reduced to 6 to 8 ng/mL. Voriconazole was stopped because all fungal cultures remained negative and because there was no evidence of aspergillus in the biopsies. Despite adequate antibiotic therapy, the patient’s clinical condition further deteriorated, he developed high fever and chills, and the CRP level rose to 252 mg/L. Pulmonary auscultation revealed crepitations over both lungs. Repeat high-resolution CT scan of the thorax indicated increase of the volume of the lung mass and lymphadenopathy and emergence of bilateral patchy pulmonary opacities. Repeat blood cultures still grew R. equi.
FIGURE 2.
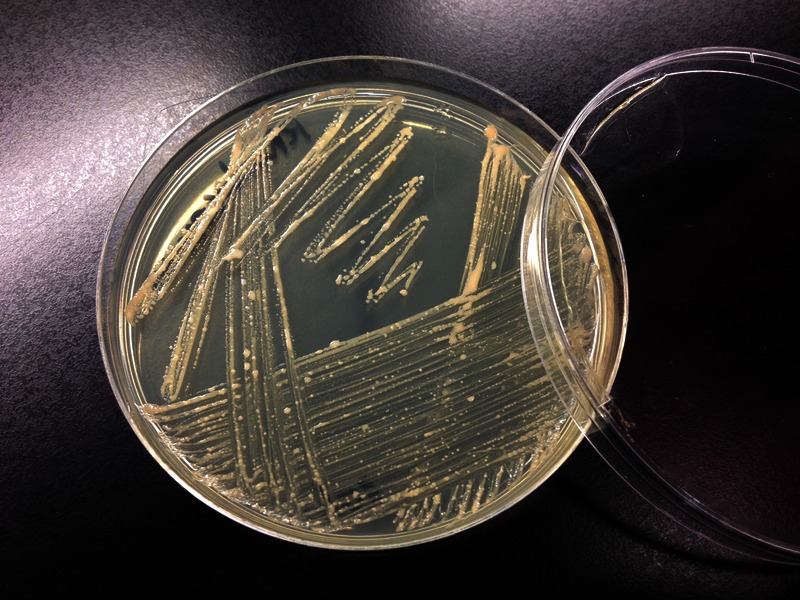
Macroscopic growth of Rhodococcus equi on nonselective TSA (tryptic soy agar).
FIGURE 3.
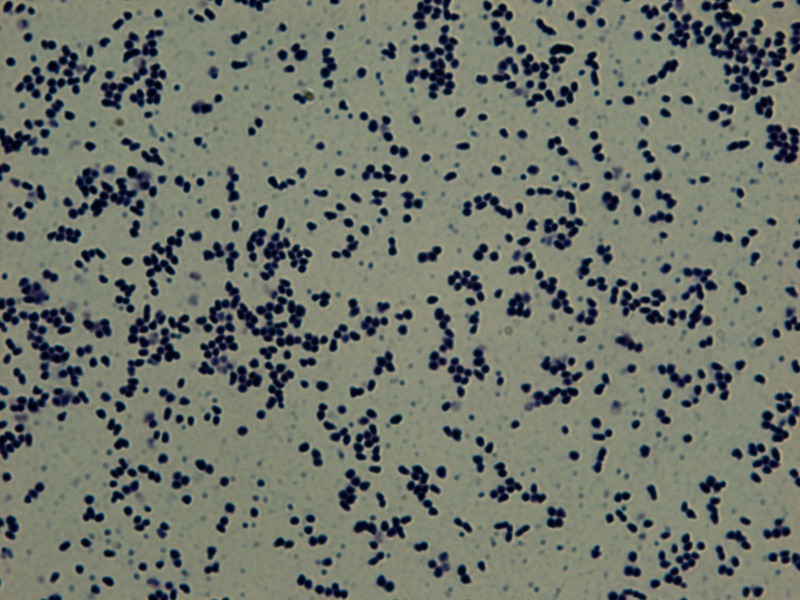
Gram staining of mucoid Rhodococcus equi colonies (amplification, 1000×).
The patient was transferred to our university hospital as a tertiary emergency care center. A third bronchoscopy with BAL was performed. The lung lesion was not accessible for ultrasound-guided transbronchial needle aspiration. Due to lack of therapeutic response, oral levofloxacin (500 mg daily) was added to the antibiotic regimen. However, the patient developed progressive hypoxic respiratory failure and was transferred to the intensive care unit. In the laboratory setting, CRP and lactate dehydrogenase levels were reported as 542 U/L and 345 mg/L, respectively. Human immunodeficiency virus (HIV) serology, plasma cytomegalovirus, and Epstein-Barr virus polymerase chain reaction tests returned negative. Repeat CT scan of the thorax showed bilateral diffuse ground glass opacity, mainly in the upper lobes (Figure 4A, B), suggestive of P. jirovecii pneumonia, and trimethoprim-sulfamethoxazole was associated. As previously mentioned, the patient was not taking any prophylaxis at that moment. The nodule in the left lower lobe had become a little smaller. Later, a positive P. jirovecii PCR in BAL fluid confirmed the diagnosis of an additional P. jirovecii pneumonia. Intravenous methylprednisolon (40 mg twice per day) was added to the therapy. No other pathogen was identified in the BAL fluid. Bronchoalveolar lavage fluid cytology showed no arguments for malignancy nor did it show any signs of pneumocystis. Levofloxacin was withdrawn because the respiratory deterioration was attributed to concomitant P. jirovecii pneumonia and in the light of repeat blood cultures that did not grow R. equi anymore. In fact, blood cultures cleared 4 days after initiation of meropenem and vancomycin (Figure 5). The patient’s vital signs remained stable, there was no need for intubation, and the oxygen supply was slowly tapered. Because of persistent hyperkalemia, trimethoprim-sulfamethoxazole was replaced by oral primaquine (26.3 mg daily)—clindamycine (600 mg, 3 times per day), which were continued for 3 weeks. Meanwhile IV therapy with meropenem and vancomycin was continued. The patient continued to improve clinically and radiologically over the subsequent weeks. In vitro testing showed susceptibility of the R. equi isolate to meropenem, levofloxacin, doxycyclin, erythromycin, gentamicin, and rifampicin. After 4 weeks of dual IV therapy, the antibiotic regimen was altered to oral moxifloxacin (400 mg daily) and rifampicin (600 mg daily). Repeat chest CT showed persistence of the lung lesion with spiculated borders in the left lower lobe, but the coexistent ground glass opacity had clearly diminished. Oral trimethoprim-sulfamethoxazole (160-800 mg 3 times a week) was continued for secondary P. jirovecii prophylaxis. The patient’s renal function restored to its baseline and CRP level was 9.9 mg/L. The patient was discharged home.
FIGURE 4.
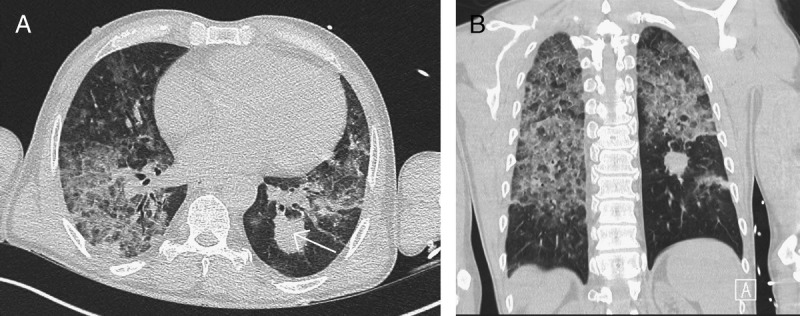
A, B, Opacity in the left lower lobe is slightly decreased in size (arrow). However, in the middle and upper parts of both lungs, large areas of dense ground glass opacity are seen now which are in many regions combined with a reticular pattern (crazy-paving pattern).
FIGURE 5.
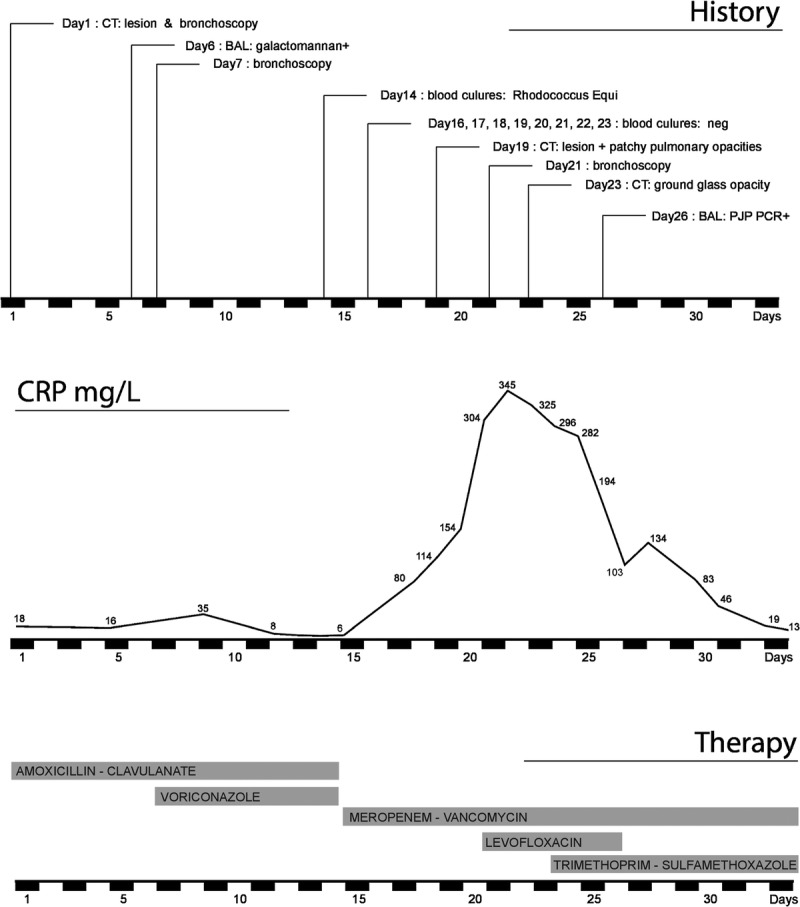
Timeline representing the relevant cultures, investigations, the biochemical evolution and the corresponding therapy.
The subsequent clinical course was favorable, although mild exertional dyspnea persisted. Serial chest CT scans showed gradual diminution of the diffuse ground glass opacities. The left lower lobe lesion first developed cavitation before its volume further decreased. Moxifloxacin and rifampicin were discontinued as planned after a total duration of 9 months of oral treatment. At present, 2 months after discontinuation of antibiotics, the patient continues to maintain timely follow-up in the outpatient setting, and clinical and radiological evolution remains favorable without signs or symptoms of recurrent infection and progressive resolution of lung lesion on chest CT scan.
DISCUSSION
Rhodococcus equi, formerly known as Corynebacterium equi, is a gram-positive, nonmotile, non–spore-forming, aerobic microorganism. It may appear as coccobacillus initially and later as bacillus, depending on growth conditions and is sometimes partially acid-fast.1 It grows on nonselective media and forms pale salmon-pink mucoid colonies after 4 to 7 days of incubation, hence its name Rhodococcus. Rhodococcus equi is a facultative intracellular pathogen; its capacity to survive in human macrophages and eventually destroy them is thought to be the basis of its pathogenesis and mechanism of antibiotic resistance and enables chronic infection.2,3 It is a soil organism that is widespread in the environment, from which it infects domesticated, grazing animals. Rhodococcus equi was originally isolated from foals in 1923 in Sweden as a cause of pyogranulomatous pneumonia4 and is now a well-known pathogen in veterinary medicine, usually causing pneumonia and sepsis, particularly in foals and cattle.5 Occasionally, R. equi causes infection in humans and the first human case was described in 1967 by Golub et al.6 In humans, R. equi infection is usually acquired by inhalation, oral ingestion, or inoculation into a wound or mucous membrane.7 Contact with animals or their manure may play a role in some reports of infection,8–10 although specific exposure was recalled in less than 50% of the cases,11 and no such exposure was reported by our patient. Rhodococcus equi infection generally affects patients who have impaired cellular immunity3, so its prevalence in humans has markedly increased over the past few decades as a result of the HIV epidemic and advances in transplantation medicine and cancer chemotherapy.4 Only 10% to 15% of R. equi infections were in seemingly immunocompetent hosts.7 Human-to-human transmission has not been documented.12,13 Our patient was taking maintenance immunosuppressive therapy after his renal transplantation. In a recent paper, Menon et al14 reviewed cases of R. equi infection in transplant recipients that were reported until 2012. They were able to collect 40 cases and concluded that most of the transplant-related R. equi infections occur after kidney transplant (58.5%) in men (82.9%) and at varying times after transplantation (ranging from 3 months to 19 years). Our patient perfectly meets these data. To the best of our knowledge, only 4 additional cases (including our case) of R. equi infection in transplant patients have been published since, 2 of them (including our case) after renal transplantation.15–17
About 80% of patients infected with R. equi develop a respiratory disease.7 In the lower respiratory tract, microorganisms are taken up by the alveolar macrophages where they grow intracellularly and cause a granulomatous inflammatory response and then cause necrosis. Later, there may be hematogenous disseminated spread. The most common radiographic finding is cavitary upper-lobe pneumonia.18 Pulmonary complications include nodules, pleural effusion, empyema, and invasion of contiguous chest structures.19 Onset of the pneumonic presentation can be insidious and may go unnoticed, particularly in immunocompromised hosts. Common symptoms are cough, pleuritic chest pain, and fever, often accompanied by constitutional symptoms such as cachexia, weight loss, and fatigue.12 Hemoptysis can also occur. Extrapulmonary sites of infection result from contiguous or hematogenous dissemination of the pathogen.20 Other described manifestations include gastrointestinal infections, pericarditis, meningitis, mastoiditis, osteomyelitis, endophtalmitis, and abscesses in brain, liver, kidney, spleen, and subcutaneous abscesses.11,21 The disease is often chronic and recurrent.
Diagnosis may be achieved by isolating the bacterium in cultures from the infection site (sputum, pleural fluid, or any other affected body tissue). To obtain these samples, bronchoscopy and sometimes more invasive procedures, such as percutaneous needle biopsy or even surgically obtained samples, are required.9,12,22 Blood cultures may also grow R. equi, and bacteremia is reported in more than half of immunocompromised patients with R. equi infection (particularly in HIV patients, in 25% of solid organ transplant recipients,2 and in 10% of immunocompetent hosts).23 Diagnosing R. equi infection is often difficult, and a delay in diagnosis is common. Because of phenotypic resemblance of the organism with other diphteroids, it may be interpreted as a contaminant. Rhodococcus equi may be misidentified as Nocardia, both from microbiological and clinical view. Nocardia too is a gram-positive aerobic microorganism with varying degrees of acid-fastness which manifests as an opportunistic infection in immunocompromised hosts and which usually manifests as pulmonary disease with subacute onset.24 Rhodococcus equi may also be mistaken for a mycobacterium because of the complex of constitutional symptoms, its insidious onset and chronic course, the organism’s predilection for the upper lung lobes, its CT findings, granuloma formation, and occasional acid-fastness,25,26 In addition, R. equi has a very slow growth rate. Rhodococcus equi can be differentiated from other pathogenic corynebacteria by its mycolic acid staining and by their inability to ferment carbohydrates.9 The characteristic histopathological features associated with Rhodococcus species are malakoplakia (an unusual type of chronic granulomatous response with Michaelis-Gutmann bodies) and necrotizing microabscesses.12,22 Recently, R. equi-specific PCR-based techniques have been developed.22 The clinical diagnosis is difficult as well: initial symptoms of R. equi disease are nonspecific, and its clinical features are many. The clinical course in our patient was fairly typical for an R. equi infection: the patient initially developed indeed a pulmonary syndrome with subacute onset and complaints of cough, hemoptysis, chest pain, and general malaise. The radiographic findings (consolidation with secondary cavitation) were also consistent with this. Diagnosis was achieved by isolating the pathogen from blood cultures. It is notable that the microorganism was not detected in repeat sputum or BAL fluid cultures, possibly because the lung lesion was located in the lower lung lobe. The lung mass was not accessible for ultrasound-guided transbronchial needle aspiration, and percutaneous needle biopsy was not performed because of response to the administered antibiotics.
There are no standardized treatment regimens for R. equi infections, and current recommendations are based on case reports and experts’ opinions. Use of bactericidal antibiotics with high intracellular penetration and concentration is the mainstay of treatment,19 and it is recommended to start with parenteral administration, followed by oral antibiotics.7,11,12 Resistance to penicillins has been reported, and therefore it is important to ascertain antibiotic susceptibility of the pathogen and to use multiple and synergistic antibiotics.27,28 Some experts recommend the combination of erythromycin and rifampicin because they act synergistically.20 Moreover, the combination of carbapenem and teicoplanin was shown to be synergistic in vitro by El Karoui et al,29 which correlated in vivo with a favorable evolution of their patient with a R. equi–associated pulmonary abscess treated with this combination. In vitro susceptibility however does not always reflect clinical effectiveness, owing to the intracellular location of the bacteria22 Rhodococcus equi is generally susceptible to macrolides, rifampicin, fluoroquinolones, vancomycin, aminoglycosides, carbapenems, and linezolid.7,12,23,30 The clinical isolate in our case was indeed resistant to penicillin, cefalosporins, piperacillin tazobactam, co-trimoxazole, and clindamycin and susceptible to meropenem, levofloxacin, doxycycline, erythromycin, gentamicin, and rifampicin. The initial unfavorable course under meropenem-vancomycin was due to a concomitant P. jirovecii pneumonia and not because of lack of response to the antibiotic regimen. The optimal duration of therapy is not known and should be individualized depending on the location and degree of tissue involvement and clinical evolution; prolonged therapy with at least 2 antibiotics is recommended.31 In addition, surgical treatment or drainage procedures are also often needed.20 In the index patient, duration of therapy was planned for an arbitrary duration of 9 months. Two months after discontinuation of antibiotic therapy, no signs of recurrence have been observed yet. A special concern in transplant patients on prolonged therapy is the potential drug interactions between the immunosuppressive therapy and the antibiotics used, such as rifampicin and erythromycin.22 Moreover, one should consider the need to drastically reduce or even cease immunosuppressive therapy.14
As previously stated, R. equi infections are often chronic, and relapses are common.9 Mortality rates range from 11% in immunocompetent patients to 20% to 25% in immunocompromised patients and 50% or more in HIV patients.12
In conclusion, we described our case to increase awareness of R. equi infections in transplant recipients, particularly when presenting with a pulmonary syndrome. Early diagnosis and prolonged therapy with multiple antibiotics guided by susceptibility testing in combination with reduction of immunosuppression may avoid unfavorable progression of this type of serious infection. A unique aspect of our case is the coinfection with P. jirovecii, which was suspected on CT scan and confirmed by PCR in BAL fluid.
Footnotes
The authors declare no funding or conflicts of interest.
REFERENCES
- 1. Rahamat-Langendoen JC, van Meurs M, Zijlstra JG, Lo-Ten-Foe JR. Disseminated Rhodococcus equi infection in a kidney transplant patient without initial pulmonary involvement. Diagn Microbiol Infect Dis. 2009; 65(4): 427– 430. [DOI] [PubMed] [Google Scholar]
- 2. Meyer DK, Reboli AC. Rhodococcus equi. In: Mandell GL, et al. Principles and Practice of Infectious Diseases. Philadelphia: Elsevier; 2005: 2472– 2478. [Google Scholar]
- 3. Prescott JF. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991; 4: 20– 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magnusson H. Spezilfische infektiose pneumonie beim fohlen: ein neuer eitererreger beim pferd. Arch Wiss Prakt Tierheilkd. 1923; 50: 22– 38. [Google Scholar]
- 5. Sebater L, Andreu H, Garcia-Valdecasas JC, et al. Rhodococcus equi infection after liver transplantation. Transplantation. 1996; 61: 980– 982. [DOI] [PubMed] [Google Scholar]
- 6. Golub B, Falk G, Spink WW. Lung abscess due to Corynebacterium equi: report of first human infection. Ann Intern Med. 1967; 66: 1174– 1177. [DOI] [PubMed] [Google Scholar]
- 7. Weinstock DM, Brown AE. Rhodococcus equi: an emerging pathogen. Clin Infect Dis. 2002; 34: 1379– 1385. [DOI] [PubMed] [Google Scholar]
- 8. Munoz P, Burillo A, Palomo J, Rodriguez-Creixems M, Bouza E. Rhodococcus equi infection in transplant recipients: case report and review of literature. Transplantation. 1998; 65: 449– 453. [DOI] [PubMed] [Google Scholar]
- 9. Perez MG, Vassilev T, Kemmerly SA. Rhodococcus equi infection in transplant recipients: a case of mistaken identity and review of the literature. Transpl Infect Dis. 2002; 4: 52– 56. [DOI] [PubMed] [Google Scholar]
- 10. Stolk-Engelaar MV, Dompeling EC, Meis JF, et al. Disseminated abscesses caused by Rhodococcus equi in a patient with chronic lymphocytic leukemia. Clin Infect Dis. 1995; 20: 478– 479. [DOI] [PubMed] [Google Scholar]
- 11. Chen XY, Xu F, Xia JY, Cheng YS, et al. Bacteremia due to Rhodococcus equi: a case report and review of literature. J Zhejiang Univ Sci B. 2009; 10(12): 933– 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kedlaya I, Ing MB, Wong SS. Rhodococcus equi infections in immunocompetent hosts: case report and review. Clin Infect Dis. 2001; 32(3): E39– E46. [DOI] [PubMed] [Google Scholar]
- 13. Gabriels P, Joosen H, Put E, et al. Recurrent Rhodococcus equi infection with fatal outcome in an immunocompetent patient. Eur J Clin Microbiol Infect Dis. 2006; 25(1): 46– 48. [DOI] [PubMed] [Google Scholar]
- 14. Menon V, Gottlieb T, Gallagher M, et al. Persistent Rhodococcus equi infection in a renal transplant patient: case report and review of the literature. Transpl Infect Dis. 2012; 14: E126– E133. [DOI] [PubMed] [Google Scholar]
- 15. Behnes CL, Neumann S, Schweyer S, et al. Pleural malakoplakia caused by Rhodococcus equi infection in a patient after stem cell transplantation. Diagn Pathol. 2012; 7: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan MY, Ali S, Baqi S. Rhodococcus equi pneumonia in a live related renal transplant recipient. J Pak Med Assoc. 2013; 63(5): 635– 638. [PubMed] [Google Scholar]
- 17. Shahani L. Rhodococcus equi pneumonia and sepsis in an allogeneic haematopoietic stem cell transplant recipient. BMJ Case Rep. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muntaner L, Leyes M, Payeras A, et al. Radiologic features of Rhodococcus equi pneumonia in AIDS. Eur J Radiol. 1997; 24: 66– 70. [DOI] [PubMed] [Google Scholar]
- 19. Speck D, Koneth I, Diethelm M, et al. A pulmonary mass caused by Rhodococcus equi infection in a renal transplant recipient. Nat Clin Pract Nephrol. 2008; 4(7): 398– 403. [DOI] [PubMed] [Google Scholar]
- 20. Johnson DH, Cunha BA. Rhodococcus equi pneumonia. Sem Respir Infect. 1997; 12: 57– 60. [PubMed] [Google Scholar]
- 21. Guyssens V1, Vandekerckhove L, Colle I, et al. Invasive infection with Rhodococcus equi—two case reports and review of literature. Acta Clin Belg. 2010; 65 (4): 271– 275. [DOI] [PubMed] [Google Scholar]
- 22. Arya B, Hussian S, Hariharan S. Rhodococcus equi pneumonia in a renal transplant patient: a case report and review of literature. Clin Transplant. 2004; 18: 748– 752. [DOI] [PubMed] [Google Scholar]
- 23. Verville TD, Huycke MM, Greenfield RA, et al. Rhodococcus equi infections in humans. 12 cases and review of literature. Medicine (Baltimore). 1994; 73: 119– 132. [DOI] [PubMed] [Google Scholar]
- 24. Wilson JW. Nocardiosis: Updates and clinical overview. Mayo Clin Proc. 2012; 87(4): 403– 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pang L-C. Pulmonary malakoplakia coexistent with turberculosis of the hilar lymph node mimicking malignancy. Respiration. 2005; 72: 95– 100. [DOI] [PubMed] [Google Scholar]
- 26. Mistry NF, Dholakia Y, D’Souza DTB, et al. Rhodococcus and Mycobacterium tuberculosis: masquerade or mixed infection. Int J Tuberc Dis. 2006; 10: 351– 353. [PubMed] [Google Scholar]
- 27. McNeil MM, Brown JM. Distribution and antimicrobial susceptibility of Rhodococcus equi from clinical specimens. Eur J Epidemiol. 1992; 8: 437– 443. [DOI] [PubMed] [Google Scholar]
- 28. Jack SS, Giguère S, Nguyen A. In vitro susceptibilities of Rhodococcus equi and other common equine pathogens to azithromycin, clarithromycin and 20 other antimicrobials. Antimicrob Agents Chemother. 2003; 47: 1742– 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. El Karoui K, Guillet C, Sekkal N, et al. Synergistic effect of carbapenem-teicoplanin combination during severe Rhodococcus equi pneumonia in a kidney transplant recipient. Transpl Infect Dis. 2009; 11: 359– 362. [DOI] [PubMed] [Google Scholar]
- 30. Kamboj M, Kalra A, Kak V. Rhodococcus equi brain abscesses in a patient without HIV. J Clin Pathol. 2005; 58: 423– 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamschchikov AV, Schuetz A, Lyon GM. Rhodococcus equi infection. Lancet Infect Dis. 2010; 10: 350– 359. [DOI] [PubMed] [Google Scholar]


