Abstract
Objectives
Diagnosis and treatment of HIV-infected mothers significantly lower rates of mother-to-child transmission (MTCT) of HIV. Early infant diagnosis (EID) is required to monitor success of prevention of MTCT (pMTCT) programmes. Our aim was to compare rates of MTCT, EID and pMTCT in countries with generalised epidemics.
Methods
The UNAIDSinfo database includes country-level information on epidemic size, prevalence of HIV infection, EID rates and pMTCT coverage. The AIDS Spectrum model was used to estimate the number of children infected with HIV in 2013, for 32 countries with generalised epidemics. Least squares linear regression, weighted by epidemic size and controlling for GDP/capita, was used to correlate national adult HIV prevalence with estimated MTCT rates.
Results
There were 32 countries with generalised epidemics included in the analysis (31 in Africa). Higher-prevalence countries (≥5%) had significantly lower rates of MTCT (P<0.01) than lower-prevalence countries (<5%). For 20 lower-prevalence countries (total 7.4 million HIV-infected people), there were 105,300 childhood (0–14 years) infections in 2013. In 12 higher-prevalence countries (total 17.1 million HIV-infected people), there were an estimated 107,500 childhood infections in 2013. Regression analysis suggests that if all countries achieved the same MTCT rate as Botswana (2.0%), childhood HIV infections could be cut by 88% (from 105,300 to 12,300 per year) in lower-prevalence countries, and by 82% (from 107,500 to 19,700 per year) in higher-prevalence countries.
Conclusions
In this analysis of 32 countries with generalised HIV epidemics, 49.5% (105,500/213,000) of childhood HIV infections in 2013 were in lower-prevalence countries. Targeting of prevention of MTCT in lower-prevalence countries needs to be prioritised, despite challenges, to reduce the number of children infected.
Keywords: MTCT, pMTCT, mother-to-child transmission, HIV, Option B+
Introduction
For HIV-infected pregnant women, without intervention, the risk of mother-to-child transmission of HIV (MTCT) ranges from 20% to 45% [1]. Antiretroviral therapy (ART) substantially reduces maternal viral load, which is the most important factor for reducing transmission (especially at delivery [2]). With ART and other interventions, at a population level, the risk of MTCT can be reduced to less than 5% for breastfeeding populations and less than 2% in non-breastfeeding populations [3]. In high-income settings, MTCT rates can be further reduced to less than 0.05% with undetectable maternal viral load (<50 copies/mL) or HIV-RNA <500–50 copies/mL combined with planned Caesarean section [2].
Programmatic distribution of ART to prevent MTCT (pMTCT) requires four critical steps: (1) diagnosis of all HIV-infected pregnant women; (2) linkage and retention in care for all those diagnosed; (3) expedited initiation of ART; and (4) sustained maternal viral suppression throughout pregnancy, delivery and breastfeeding. This continuum of care is known as the HIV treatment cascade for pMTCT. In low-income and high-prevalence settings, pMTCT success may be more difficult. However, interim recent results of the Promoting Maternal-Infant Survival Everywhere (PROMISE) trial, ongoing in India, Malawi, South Africa, Tanzania, Uganda, Zambia and Zimbabwe, report that MTCT rates can be reduced to 0.56% [4].
It has been known since the early 1990s that control of viral replication using monotherapy during pregnancy can reduce the risk of transmission to children significantly [5]. This became a priority of HIV prevention programmes, known as Option A (see Box 1). Following the successes of pioneered treatment guidelines by the Malawian Ministry of Health [6], the 2011 UNAIDS Global Plan to eliminate new HIV infections among children and keep their mothers alive, was set for 2015. The UNAIDS Global plan covers all low- and middle-income countries, but focuses on 22 countries (Angola, Botswana, Burundi, Cameroon, Chad, Côte d'Ivoire, Democratic Republic of Congo, Ethiopia, Ghana, India, Kenya, Lesotho, Malawi, Mozambique, Namibia, Nigeria, South Africa, Uganda, United Republic of Tanzania, Swaziland, Zambia and Zimbabwe) with the highest estimate of HIV-infected pregnant women.
Box 1. Summary of guidelines for the treatment of HIV-infected mothers and their children, and definitions used.
| Mother | Child | ||
|---|---|---|---|
| Option A | If CD4 cell count ≤350 cells/μL, start triple antiretrovirals at diagnosis and continue for life. If CD4 cell count >350 cells/μL start antepartum zidovudine at 14 weeks' gestation. Intrapartum: standard dose nevirapine, zidovudine and lamivudine; postpartum: zidovudine and lamivudine for 7 days | Standard dose nevirapine daily for 6 weeks in non-breastfed infants or with mothers on antiretroviral therapy, or until 1 week after all breastfeeding has stopped | |
| Option B | All HIV-infected pregnant women start triple antiretrovirals irrespective of CD4 cell count. If CD4 cell count ≤350 cells/μL, continue triple ART for life. If CD4 cell count >350 cells/μL, start triple antiretrovirals at 14 weeks' gestation and continue intrapartum and through childbirth. Stop if mother is not breastfeeding or continue until 1 week after all breastfeeding has stopped | Daily nevirapine or zidovudine from birth to 4–6 weeks | |
| Option B+ | All HIV-infected pregnant women start on triple antiretrovirals irrespective of CD4 cell count and continue for life | Daily nevirapine or zidovudine from birth to 4–6 weeks | |
| Definitions | |
|---|---|
| MTCT rate | Estimated mother-to-child transmission rate of HIV. The estimated percentage of infants born to HIV-infected mothers who are diagnosed with HIV by 12 months |
| pMTCT coverage | The estimated coverage of prevention of mother-to-child transmission of HIV programmes. The estimated percentage of pregnant women living with HIV who received antiretrovirals for the prevention of mother-to-child transmission of HIV (Options A, B or B+), but excluding single-dose nevirapine only |
| Childhood HIV incidence | The estimated number of children <14 years old who are newly infected with HIV per year |
| Early infant diagnosis (EID) testing | The percentage of infants tested for HIV nucleic acid within the first 2 months of life |
In the UNAIDS Global Plan, elimination of MTCT is defined by four criteria: (1) reducing infant incidence to under 50/100,000 live births; (2) MTCT rate <5% in breastfeeding populations and <2% in non-breastfeeding populations; (3) >95% of all pregnant women receive ≥1 antenatal visit and know their HIV status; (4) ≥95% of HIV-infected women receive ART, which must be maintained for over 12 months.
In 2013, the WHO guidelines for MTCT shifted to recommend the initiation of ART for all pregnant and breastfeeding women with HIV, regardless of CD4 cell count, and continuation on ART for life (known as Option B+, Box 1). This simplified delivery of pMTCT services programmatically, and many countries transitioned straight from option A to option B+. High coverage at all stages of the mother cascade is required for elimination of MTCT and this is influenced by individual, structural and socio-cultural factors [7].
As of August 2015, only Cuba had successfully achieved WHO-validated elimination of pMTCT with just two vertical transmissions in 2013. In contrast, despite 63,000 HIV infections being successfully prevented amongst infants due to successful pMTCT in South Africa, an estimated 12,000 children (<14 years) acquired HIV in South Africa alone, the vast majority being via MTCT. In the 33 countries included in this analysis, 213,000 children (<14 years) acquired HIV in 2013 and a total of 2,844,800 children (<14 years) were living with HIV in these countries.
In this analysis, we aimed to compare estimated successes of pMTCT programmes in 32 high- and low-burden countries with generalised epidemics, using data for 2013.
Methods
The UNAIDS 2013 database, includes estimates of adult HIV prevalence and the percentage of pregnant mothers taking antiretroviral treatment. The UNAIDS Spectrum model was used to estimate the total number of children newly infected with HIV in 2014 in each country. We used data from 32 low- and middle-income countries with total generalised HIV epidemics of at least 40,000 people. Countries with focused epidemics amongst high-risk groups only (e.g. people who inject drugs or men who have sex with men) were excluded from this analysis, because estimates of total new HIV infections in children could not be estimated reliably from the data available.
Least squares linear regression, weighted by total adult HIV epidemic size and controlling for GDP per capita, was used to correlate national level prevalence of HIV with estimated rates of MTCT.
WHO guidelines indicate to continue breastfeeding exclusively for 6 months and throughout weaning to 12 months, for infants born to HIV-infected mothers on successful treatment [8], which means pMTCT programmes must prioritise reducing loss to follow-up amongst breastfeeding mothers [1].
The definition of MTCT used in this analysis was the percentage of all children born to HIV-infected mothers who had been infected with HIV by 12 months of age. This will include infants born from undiagnosed and/or untreated HIV-infected mothers. The MTCT rate reflects the true effectiveness of the pMTCT programme from 12–21 months prior to the endpoint (2013), due to the 9-month gestational period. The definition of pMTCT was the estimated percentage of pregnant women living with HIV who received antiretrovirals for the prevention of mother-to-child transmission of HIV (Options A, B or B+), but excluding single-dose nevirapine only.
Excluded from the analysis of MTCT rates were infants infected through breastfeeding post 12 months through mothers seroconverting postpartum. While this may not represent the majority of incidence in <14 year olds, there is a spike in viral load at seroconversion [9] that can be highly infectious to breastfeeding infants. Data used in this analysis is also an estimation to an extent, because results cannot be easily collected for mothers and infants who are never tested for HIV, or those who never registered as pregnant, or who miscarried, or infants who die early or whose births are not registered.
We also compared data on early infant diagnosis (EID; defined as a test 2 months post delivery) coverage, with adult HIV prevalence, weighted for epidemic size in children <15 years, for the same 32 countries. Furthermore, reported coverage of pMTCT programmes was compared with the MTCT rates.
Results
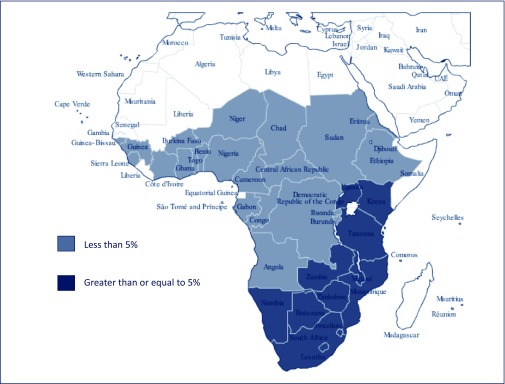
Figure 1 shows the countries included in this analysis. The countries with adult HIV prevalence less than 5% were in Central or Western Africa and Haiti, while the countries with prevalence at or above 5% were in Eastern or Southern Africa.
Figure 1.
Map showing countries in Africa included in the analysis, by adult prevalence of HIV
Tables 1 and 2 show the estimated number of adults infected with HIV in each country, and the number of new HIV infections in children in 2013. Tables 3 and 4 show other measures of treatment and care in children, by country: the percentage of HIV-infected mothers receiving antiretroviral treatment during pregnancy, the percentage of children who received an early infant diagnosis test, and the percentage of children receiving antiretroviral treatment.
Table 1.
HIV infections in children from countries with adult HIV prevalence <5% (UNAIDS 2013 database)
| Country | Epidemic size (adults, n) | Adult HIV prevalence (%) | MTCT rate (%) | New child infections (n) |
|---|---|---|---|---|
| Burkina Faso | 110,000 | 0.9% | 22% | 1,200 |
| Burundi | 83,000 | 1.0% | 25% | 1,300 |
| Benin | 74,000 | 1.1% | 22% | 1,000 |
| DR Congo | 440,000 | 1.1% | 29% | 7,400 |
| Ethiopia | 790,000 | 1.2% | 25% | 8,300 |
| Ghana | 220,000 | 1.3% | 21% | 2,400 |
| Sierra Leone | 57,000 | 1.6% | 19% | 1,000 |
| Guinea | 130,000 | 1.7% | 22% | 1,400 |
| Haiti | 140,000 | 2.0% | 8% | 500 |
| South Sudan | 150,000 | 2.2% | 31% | 2,600 |
| Togo | 110,000 | 2.3% | 18% | 1,100 |
| Angola | 250,000 | 2.4% | 25% | 4,000 |
| Chad | 210,000 | 2.5% | 32% | 3,700 |
| Congo | 69,000 | 2.5% | 34% | 1,000 |
| Cote d'Ivoire | 370,000 | 2.7% | 23% | 4,900 |
| Nigeria | 3,200,000 | 3.2% | 26% | 51,000 |
| Guinea-Bissau | 41,000 | 3.7% | 24% | 1,000 |
| CAR | 120,000 | 3.8% | 32% | 1,500 |
| Gabon | 41,000 | 3.9% | 14% | 500 |
| Cameroon | 600,000 | 4.3% | 25% | 9,500 |
| Total | 7,205,000 | 105,300 |
Table 2.
HIV infections in children from countries with adult HIV prevalence ≥5% (UNAIDS 2013 database)
| Country | Epidemic size (adults, n) | Adult HIV prevalence (%) | MTCT rate (%) | New child infections (n) |
|---|---|---|---|---|
| Tanzania | 1,400,000 | 5.0% | 16% | 16,000 |
| Kenya | 1,600,000 | 6.0% | 16% | 13,000 |
| Uganda | 1,600,000 | 7.4% | 13% | 16,000 |
| Malawi | 1,000,000 | 10.3% | 13% | 7,400 |
| Mozambique | 1,600,000 | 10.8% | 12% | 12,000 |
| Zambia | 1,100,000 | 12.5% | 15% | 12,000 |
| Namibia | 250,000 | 14.3% | 10% | 1,100 |
| Zimbabwe | 1,400,000 | 15% | 13% | 9,000 |
| South Africa | 6,300,000 | 19.1% | 6% | 16,000 |
| Botswana | 320,000 | 21.9% | 2.0% | 500 |
| Lesotho | 360,000 | 22.9% | 22% | 3,400 |
| Swaziland | 200,000 | 27.4% | 10% | 1,100 |
| Total | 17,130,000 | 107,500 |
Table 3.
Antiretroviral treatment in countries with adult HIV prevalence <5% (UNAIDS 2013 database)
| Country | Children with HIV (n) | pMTCT (%) | EID testing (%) | Children on ART (%) |
|---|---|---|---|---|
| Burkina Faso | 18,000 | 62% | 26% | 10% |
| Burundi | 18,000 | 58% | 17% | 12% |
| Benin | 8,400 | 45% | 18% | 16% |
| DR Congo | 66,000 | 33% | 10% | 8% |
| Ethiopia | 200,000 | 55% | 21% | 9% |
| Ghana | 35,000 | 62% | 30% | 11% |
| Sierra Leone | 5,000 | 93% | 41% | 8% |
| Guinea | 13,000 | 46% | 5% | 10% |
| Haiti | 13,000 | 93% | 37% | 20% |
| South Sudan | 18,000 | 16% | n.d. | 2% |
| Togo | 21,000 | 75% | 14% | 16% |
| Angola | 29,000 | 39% | 18% | 14% |
| Chad | 34,000 | 19% | 4% | 5% |
| Congo | 13,000 | 23% | 9% | 9% |
| Cote d'Ivoire | 72,000 | 75% | 15% | 8% |
| Nigeria | 400,000 | 27% | 4% | 12% |
| Guinea-Bissau | 6,100 | 56% | 6% | 7% |
| CAR | 17,000 | 33% | 4% | 5% |
| Gabon | 4,000 | 62% | 22% | 18% |
| Cameroon | 94,000 | 61% | 24% | 6% |
n.d.: no data
Table 4.
Antiretroviral treatment for countries with adult HIV prevalence ≥5% (UNAIDS 2013 database)
| Country | Children with HIV | pMTCT (%) | EID testing (%) | Children on ART (%) |
|---|---|---|---|---|
| Tanzania | 250,000 | 73% | 26% | 16% |
| Kenya | 190,000 | 63% | 42% | 31% |
| Uganda | 190,000 | 75% | 37% | 22% |
| Malawi | 170,000 | 79% | 15% | 24% |
| Mozambique | 190,000 | 84% | 35% | 22% |
| Zambia | 150,000 | 76% | 55% | 33% |
| Namibia | 23,000 | 90% | 56% | 45% |
| Zimbabwe | 170,000 | 78% | 50% | 27% |
| South Africa | 360,000 | 90% | 78% | 44% |
| Botswana | 11,000 | 95% | 58% | 84% |
| Lesotho | 36,000 | 53% | 36% | 15% |
| Swaziland | 1,100 | 95% | 89% | 46% |
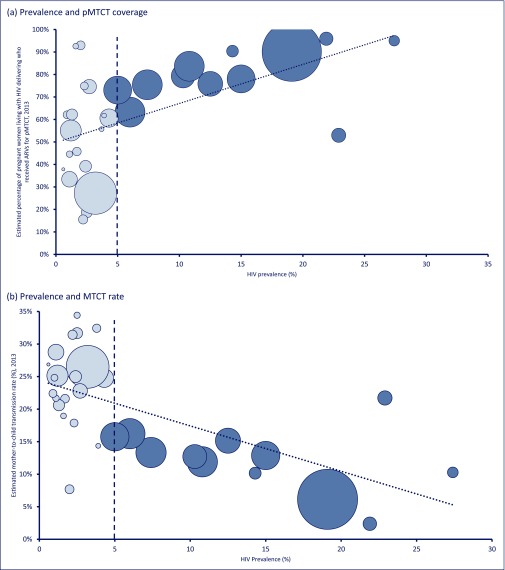
Figure 2(a) shows the percentage of mothers receiving antiretroviral treatment during pregnancy (pMTCT). There was a clear trend for higher percentages of women receiving pMTCT in countries with a higher adult prevalence of HIV.
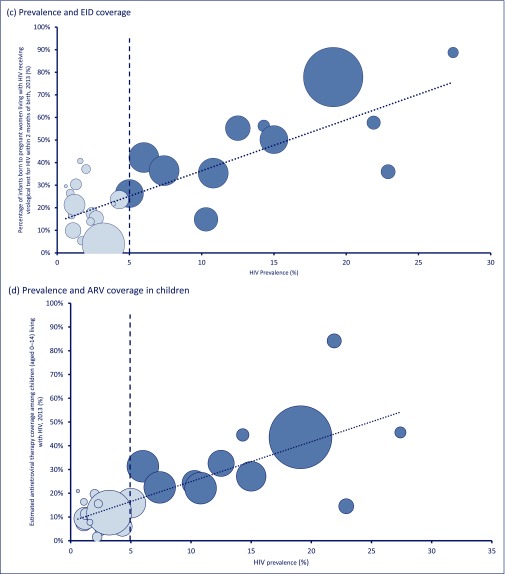
Figure 2.
Prevalence of HIV in adults versus measures of treatment and care in children. Prevalence and (a) pMTCT coverage; (b) MTCT rate; (c) early infant diagnosis coverage; (d) ARV coverage in children. The size of the circles represents the size of the epidemic in each country. Countries with a prevalence <5% have lighter shading
Figure 2(b) illustrates that, as the total prevalence of HIV increases, the MTCT rate decreases. This would be expected from the analysis of pMTCT, shown in Figure 2(a). Of the 32 countries with generalised epidemics included in this analysis, Swaziland had the highest prevalence of HIV (27.4%) and had an MTCT rate of 10%, while Burkina Faso had the lowest prevalence (0.9%) and an MTCT rate of 22%. The lowest MTCT rate was in Botswana, where 2% of infants born to HIV-infected mothers had HIV at 12 months and pMTCT coverage was over 90% (Figure 2). The highest MTCT rates were in: Congo (34%); Chad (32%); Central African Republic (32%); South Sudan (31%); and the Democratic Republic of Congo (29%) where prevalence was relatively lower (2.5%, 2.5%, 3.8%, 2.2% and 1.1%, respectively). Following the weighted regression analysis controlling for GDP, as adult HIV prevalence increases by 10%, the MTCT rate in a country reduces by 5.83%. The size of the circle representing a country is proportional to the number adults living with HIV.
A total of 213,000 childhood (<14 years) infections occurred in the 32 countries included in this analysis in 2013. Twelve countries with high prevalence (≥5%) were responsible for 50.5% of these cases, while the other 49.5% of these new infections occurred in the 20 low-prevalence countries. The regression analysis suggests that, if all countries could achieve the same MTCT rate as Botswana (2%), HIV infections in children could be cut by 88% (from 105,500 to 12,300 per year) in lower prevalence countries, and by 82% (from 107,500 to 19,700 per year) in higher prevalence countries.
Figure 2(c) shows the coverage of virological testing, by 2 months post delivery, for infants born to diagnosed HIV-infected mothers or EID. Figure 2(c) shows that the percentage of infants born to diagnosed HIV-infected mothers receiving EID by 2 months increases as the total adult HIV epidemic size increases across 32 countries with generalised epidemics. As total HIV prevalence increases between countries by 10%, EID coverage increases by about 22%. This means that, generally, countries with a higher adult HIV prevalence (e.g. Swaziland, highest at 27.4%) have higher coverage of EID (highest, at 88.75%), while countries with low adult HIV prevalence (e.g. Central African Republic, 3.8%) have poor coverage of EID (lowest at 3.6%).
Figure 2(d) shows the percentage of children receiving antiretroviral treatment by country, according to prevalence of HIV in adults. This shows the same trend as the previous analyses, with very low percentages of children receiving antiretroviral treatment in the low-prevalence countries.
Discussion
In this analysis of 32 countries with generalised HIV epidemics, the risk of mother-to-child transmission of HIV was highest in countries with the lowest adult prevalence of HIV. Early treatment initiation, regardless of CD4 cell count, has been found protective for individual health and can reduce population-level transmission [10,11]. However, for HIV-infected pregnant women ART initiation needs to be expedited, allowing sufficient time for sustained viral suppression by the time of delivery. In addition, due to the high birth rate of women living in resource-limited settings and longer breastfeeding times, sustainable delivery of ART to postpartum women who continue to breastfeed until their subsequent pregnancy must be implemented.
The relationships presented in this analysis, that between 32 countries with generalised epidemics an average 10% increase in pMTCT coverage is related to a 3 to 3.5% reduction in MTCT rate and a 22% increase in EID coverage, are similar to the model by Tanser et al., where in South African high-prevalence communities, an increase by 1% for ART coverage led to an average 1.4% decrease in the risk of acquiring HIV [12].
Some countries were outliers from the observed trends. Of particular concern was the high MTCT rate (22%) and low EID coverage (39%) in Lesotho, where the prevalence of HIV was high (22.9%) although similar to Botswana. The pMTCT coverage is much lower in Lesotho than in Botswana, which may explain this.
Our results show differences between countries that may be explained by national guidelines and procedures. For example, South Africa achieves a much higher coverage of EID than our modelled relationship would predict, or any other country except for Swaziland. This may be influenced by the fact that South Africa encourages immediate HIV testing at birth in many settings [13].
Many of the countries analysed in this paper had very recently begun implementing the shift in national pMTCT guidelines towards Option B+ either directly from Option A or from Option B; however, this process is not immediate or uniform, and in some countries it takes longer than others. Furthermore, there is a range of pMTCT effectiveness even within a country and regional variability is not taken into account in our analysis. In Swaziland, Option B+ was implemented at the end of 2012 and the percentage of HIV-infected mothers receiving ART rose from 32% to 56% between 2011 and 2013. Comparatively, in parts of Mozambique where coverage was <10% in 2011, when guidelines changed in 2013 coverage rose to >80% [14]. Time to successful implementation of national guidelines can vary from a few months to several years and coverage may not be uniform across each country, therefore, attributing success to guideline changes or programmatic implementation is difficult.
Reducing MTCT rates to below 2% may seem a long way away for some countries, but we know it has been achieved in Botswana in a short period of time, and clinical trials in many low-income, high-burden, generalised epidemic settings report MTCT rates of 0.56% [4]. Even countries like Botswana, which have low rates of mother-to-child transmission, do not yet have high rates of early infant diagnosis testing. Without high rates of EID, children may not be treated early enough to prevent HIV disease progression and death.
With the current national MTCT data it cannot be assessed on a country-wide level whether infections primarily occur prenatally or via breastfeeding; however, this data would be important for countries in order to focus resources and improve their pMTCT programmes. In a meta-analysis of African cohorts, MTCT risk was significantly higher among women with incident versus chronic HIV infection in the postpartum period or in pregnancy/postpartum periods combined [9].
Since Malawi pioneered Option B+ in antenatal care services for women, the number of pregnant women living with HIV who are accessing treatment increased by 748% [6]. However, even in Malawi, about 15% of women do not initiate ART or become lost to follow-up within 6 months [5]. A study from Nigeria and Malawi found that the main barriers to successful pMTCT coverage were socio-cultural and socio-economic factors but also flaws in pMTCT programmatic designs, limited male-partner involvement and healthworker insufficiencies [7]. In the PROMISE trial [4], triple combination treatment of pregnant women was associated with significantly lower rates of mother-to-child transmission of HIV. The results from the PROMISE trial reinforce the importance and success of Option B+ in high-burden, low-income settings [4].
Conclusion
In conclusion, countries with a lower prevalence of HIV (below 5%) need to be prioritised for more intensive treatment and care of HIV-infected mothers and their children. In this analysis, rates of mother-to-child transmission are particularly high in these countries, and the children born HIV infected in these countries tend not to be diagnosed or treated.
Acknowledgements
Andrew Hill and Carmen Pérez Casas designed the project. Jacob Levi and Thomas Dauncey wrote the manuscript. Katherine Heath analysed the data.
The authors declare no conflict of interest.
Funding was provided by UNITAID.
References
- 1. Stevens J, Lyall H. Mother to child transmission of HIV: what works and how much is enough? J Infect 2014; 69 Suppl 1: S56– 62. [DOI] [PubMed] [Google Scholar]
- 2. Townsend CL, Byrne L, Cortina-Borja M et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS 2014; 28: 1049– 1057. [DOI] [PubMed] [Google Scholar]
- 3. De Cock KM, Fowler MG, Mercier E et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA 2000; 283: 1175– 1182. [DOI] [PubMed] [Google Scholar]
- 4. Fowler M, Qin M, Shapiro D. PROMISE: efficacy and safety of 2 strategies to prevent perinatal HIV transmission. Conference on Retroviruses and Opportunistic Infections. February 2015. Seattle, WA, USA. Abstract 31LB.
- 5. Cooper ER, Charurat M, Burns DN et al. Trends in antiretroviral therapy and mother-infant transmission of HIV. The Women and Infants Transmission Study Group. J Acquir Immune Defic Syndr 2000; 24: 45– 47. [DOI] [PubMed] [Google Scholar]
- 6. Herce ME, Mtande T, Chimbwandira F et al. Supporting Option B+ scale up and strengthening the prevention of mother-to-child transmission cascade in central Malawi: results from a serial cross-sectional study. BMC Infect Dis 2015; 15: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okoli JC, Lansdown GE. Barriers to successful implementation of prevention-of-mother-to-child-transmission (PMTCT) of HIV programmes in Malawi and Nigeria: a critical literature review study. Pan Afr Med J 2014; 19: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization PMTCT strategic vision 2010–2015: preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals. Moving towards the elimination of paediatric HIV. Available at: www.who.int/hiv/pub/mtct/strategic_vision.pdf ( accessed September 2015).
- 9. Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med 2014; 11: e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lundgren JD, Babiker AG, Gordin F et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015; 373: 795– 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen MS, Chen YQ, McCauley M et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493– 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanser F, Barnighausen T, Grapsa E et al. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013; 339: 966– 971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lilian RR, Johnson LF, Moolla H, Sherman GG. A mathematical model evaluating the timing of early diagnostic testing in HIV-exposed infants in South Africa. J Acquir Immune Defic Syndr 2014; 67: 341– 348. [DOI] [PubMed] [Google Scholar]
- 14. Kieffer MP, Mattingly M, Giphart A et al. Lessons learned from early implementation of option B+: the Elizabeth Glaser Pediatric AIDS Foundation experience in 11 African countries. J Acquir Immune Defic Syndr 2014; 67 Suppl 4: S188– 194. [DOI] [PMC free article] [PubMed] [Google Scholar]