Abstract
Until recently, hepatitis E was thought to be largely confined to hyperendemic areas in Asia, Africa and Mexico. Over the last 10 years it has become clear that this is not the case, as it is surprisingly common in developed countries. In these settings, it is caused by HEV genotypes 3 and 4, and is a porcine zoonosis. It causes a range of human illness including acute and chronic hepatitis, and a spectrum of neurological injury. HEV RNA has been found in donated blood from an increasing number of countries, and in some locations with a very high incidence. The clinical phenotype and burden of disease in humans is still emerging. In contrast to previous ‘received wisdom’, zoonotically transmitted HEV may be one of the most successful zoonotic viral infections in human history. How did we, as a scientific community, get this so badly wrong? This review considers this question from a largely clinical perspective, explores the places HEV has been ‘hiding’ and the emerging clinical phenotype in humans.
Keywords: hepatitis E virus (HEV), hepatitis, zoonosis, neurological injury, blood products
Hepatitis E in developing countries
For many years hepatitis E virus (HEV) was thought to be largely confined to the developing world where it is a major health issue often causing large outbreaks. In such settings hepatitis E is caused by HEV genotypes 1 and 2, which are obligate human pathogens spread orofaecally via contaminated water supplies [1,2]. It mainly affects young adults with a self-limiting illness, except in pregnant women where fulminant hepatic failure can occur, with a high mortality rate [3].
A recent study has estimated that in nine of 21 global burden of disease (GBD) regions, there are 3.4 million symptomatic cases of hepatitis E each year, with 70,000 deaths and 3,000 stillbirths [4]. This is almost certainly an underestimate of the GBD, as there may be up to 1,000 HEV-related maternal deaths per annum in Bangladesh alone [5]. In addition, recent studies suggest that anti-HEV seroprevalence estimates (on which the GBD was partly calculated) may have lacked sensitivity, and underestimated the true seroprevalence by 50% (unpublished observations).
Hepatitis E in developed countries
Following the discovery of HEV in the 1980s in developed countries, hepatitis E was thought to be a disease seen only in travellers returning from endemic areas in the developing world. Hepatitis E was considered exceedingly rare and of little relevance. Conceptually, the virological and hepatological features of HEV were considered analogous to hepatitis A virus (HAV): both being orofaecal hepatotropic RNA viruses causing acute self-limiting hepatitis. These misguided notions unfortunately remained the ‘received wisdom’ for the best part of 20 years [6].
Acute hepatitis E
We have been studying HEV in southwest England for over 10 years [6–21]. In epidemiological terms, this is an ideal setting to study an autochthonous disease such as hepatitis E owing to: (a) its geographical isolation; (b) the very low number of immigrants born overseas (crucial in the context of study of locally acquired infection); (c) a very limited number of secondary care providers; and (d) the existence of rapid-access jaundice clinics (the Jaundice Hotline Clinic, JHL) [22].
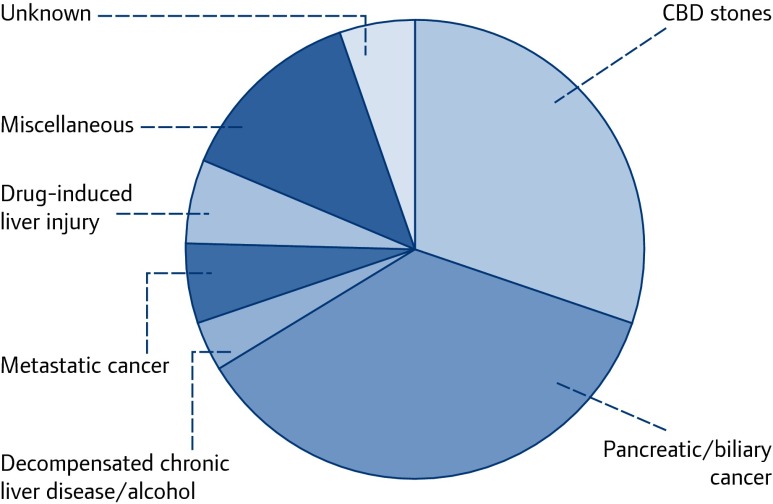
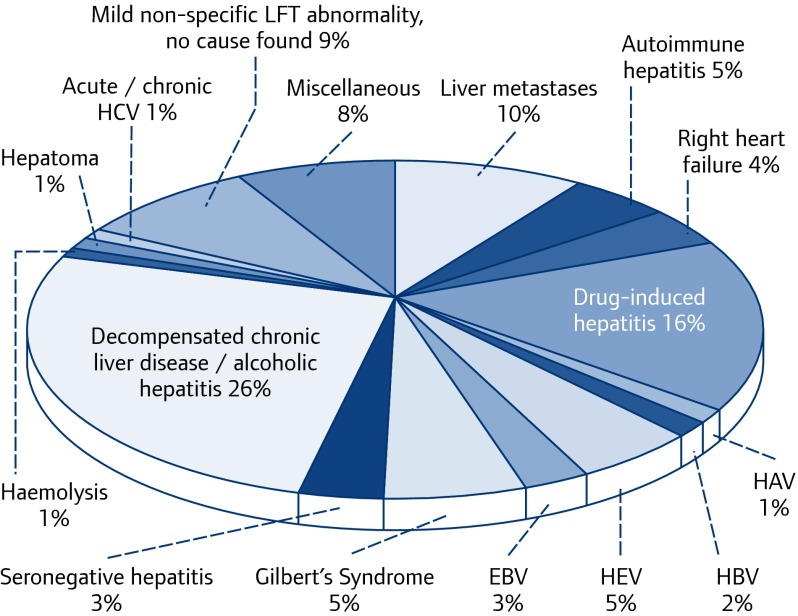
The JHL has proven to be an invaluable tool for studying the epidemiology and clinical features of acute autochthonous hepatitis E, as most patients developing acute jaundice/hepatitis in our community are reviewed via the JHL, and laboratory and clinical outcome data are prospectively recorded [23–25]. Initially we noted that, despite appropriate investigation, there were a number of patients with hepatitis for whom we had no diagnosis (Figure 1). Such patients were retrospectively and then prospectively tested for HEV. Recent analysis shows that autochthonous hepatitis E accounts for 5% of patients presenting with hepatocellular jaundice to the JHL (Figure 2), and is the commonest cause of acute hepatitis in southwest England by quite a margin (Table 1) [23–25].
Figure 1.
Final diagnostic category in patients aged >60 years from consecutive patients presenting to the Jaundice Hotline Clinic, Cornwall UK, 1998–2003. Despite appropriate investigation, a minority had no diagnosis
Figure 2.
Final diagnoses in 1054 consecutive patients with jaundice/hepatitis presenting in the Jaundice Hotline Clinic 1998–2014, showing that 5% are caused by HEV genotype 3
Table 1.
Causes of acute viral hepatitis
| Incidence* (%) | Comments | |
|---|---|---|
| HEV | 5 | Affects predominantly middle–aged and elderly males |
| Seronegative hepatitis [24] | 3 | Negative for all known causes of acute viral hepatitis |
| Occurs at all ages, including adolescents | ||
| May be caused by an as yet unidentified hepatotropic virus | ||
| EBV [25] | 3 | Surprisingly common, occurs at all ages |
| Mild hepatitis, but can be severe in elderly | ||
| <10% have symptoms of infectious mononucleosis | ||
| Diagnosis suggested by a combination of hepatitis, splenomegaly and lymphocytosis, which is present in 95% | ||
| HBV | 2 | ALT is usually higher (3–500 IU/L) than in HEV |
| HCV | <1 | Very uncommon |
| CMV | <1 | Very uncommon |
Incidence refers to the percentage of individuals with each infection from a cohort of 1,054 consecutive patients presenting to the JHL Clinic, Cornwall, UK 1998–2014.
HEV: hepatitis E; HAV: hepatitis A; HBC: hepatitis B; HCV: hepatitis C; CMV: cytomegalovirus; EBV: Epstein–Barr virus; ALT: alanine transaminase.
We have now carefully documented over 100 cases of acute autochthonous hepatitis E [26]. All were caused by HEV genotype 3, when sequencing was possible. Remarkably, and uniquely, acute hepatitis E appears to have a predilection for middle-aged/elderly males [1], with a male:female ratio of 3.2:1 and a median age of 63.5 years [26]. Acute hepatitis E caused by genotype 3 (and genotype 4 in China, Japan and a few recently described clusters in Europe) [27,28] has been found in every single developed country in which it has been sought. All these studies have described very similar demographics, with an excess of cases in older men [13,14,29–33]. The reasons for these observations are uncertain.
The symptoms of hepatitis E infection are similar to those seen in any form of viral hepatitis (Table 2) [26], except in a minority of patients who present with a primarily neurological illness (see later). A number of other extra-hepatic manifestations have been described (Table 3) [20,26,34–37]. In contrast to HEV genotype 1, excess mortality in pregnant women is not seen with genotype 3, and the few women who have been described in the literature have all survived [38]. The majority of patients have a self-limiting illness, with clinical and biochemical recovery within a few weeks. A minority have a more severe hepatitis, and some patients (3.8% in our recent series) [26] die from subacute liver failure [16,19,21,39]. Such patients usually have underlying chronic liver disease [19,40].
Table 2.
Top 10 most common symptoms of acute HEV in our cohort in southwest England [26]
| Symptom | Patients with symptom (%) | |
|---|---|---|
| 1 | Jaundice | 59 |
| 2 | Malaise / lethargy | 34 |
| 3 | Nausea and vomiting | 29 |
| 4 | Abdominal pain | 26 |
| 5 | Loss of appetite | 23 |
| 6 | Myalgia | 14 |
| 7 | Fever | 13 |
| 8 | Loss of weight | 10 |
| 9 | Asymptomatic | 9 |
| 10 | Neurological | 8 |
Table 3.
Extrahepatic manifestations of acute and chronic hepatitis E
Studies of acute HEV genotype 1 infection in patients with chronic liver disease in Asia show a mortality rate of up to 70% [41]. A prospective UK/French study of 372 patients with decompensated chronic liver disease and HEV genotype 3 showed a mortality rate of 27% (Blasco-Perrin et al., personal communication). The incidence of hepatitis E in the context of chronic liver disease varies significantly by geographical location, and was much more common in southwest France (7.9%) than in the UK (1.1%). There appears to be no clinical or laboratory clues to diagnosis at presentation, and so patients with decompensated chronic liver disease should be considered for routine HEV testing, particularly in high incidence areas. An early diagnosis is important, as patients with chronic liver disease who have decompensated due to HEV infection have been successfully treated with ribavirin [19].
Chronic hepatitis E
The field of hepatitis E was changed for ever by the description of chronic infection in transplant recipients in two side-by-side papers from southern France published in the New England Journal of Medicine in 2008 [42,43]. Typically patients have no symptoms, they are not jaundiced and their alanine aminotransferase (ALT) runs between 200 and 300 IU/L. Chronic infection occurs in approximately 60% of solid organ transplant recipients exposed to HEV genotype 3 infection [44]. Progressive liver disease is common and is usually more rapid than that seen in chronic infection with HBV or HCV and 10% of recipients with chronic hepatitis E infection are cirrhotic within 2 years [44,45]. The prevalence of chronic hepatitis E in the European transplant population averages between 1% and 2% [46,47]. The figure is much higher in the transplant centres in southwest France [44].
Chronic hepatitis E infection can also occur in other immunosuppressed groups, including patients with haematological malignancy [48] and in individuals with HIV infection [12]. In the latter group, HIV/HEV chronic co-infection is uncommon, and is only seen in patients who are profoundly immunosuppressed with CD4 cell counts <250 cells/μL. So far chronic infection has only been described with HEV genotype 3 [49].
Treatment and prevention
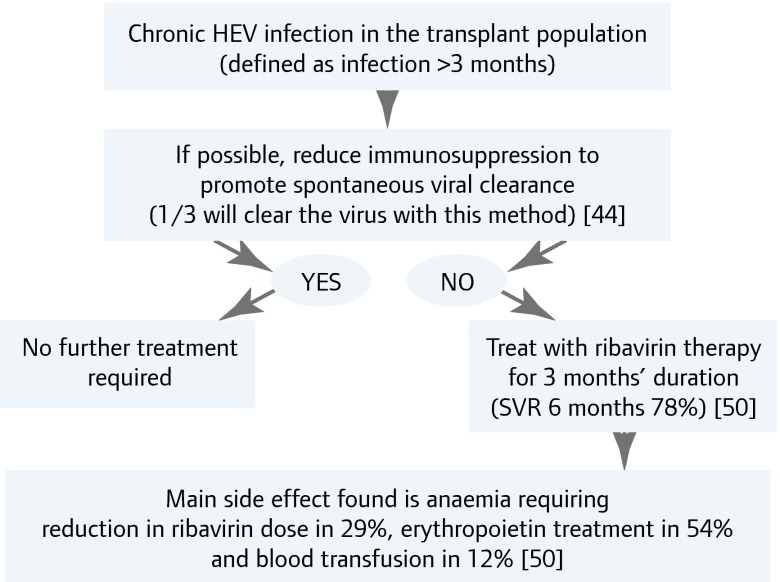
Case reports and case series show that chronic hepatitis E infection can be treated with the antiviral agents ribavirin and/or interferon. Most published data concern treatment of chronically infected solid-organ transplant recipients with ribavirin. The suggested treatment algorithm is shown in Figure 3 [50]. Acute infection generally requires no treatment, as it is usually a self–limiting illness. A few cases of severe hepatitis have been treated successfully with ribavirin (see above) [21].
Figure 3.
Treatment algorithm for chronic HEV infection in the transplant population
A safe and effective vaccine has now been licensed for use in China [51]. It is not known whether it will be licensed for use in other countries.
Epidemiology
The incidence of hepatitis E infection varies between and also within countries. The incidence in the USA is 0.7% [52], the Netherlands 1.1% [53] and in southwest France it is as high as 3.2% [54]. In the UK, the incidence is 0.2% [2]. As in France, where infection is more common in the south of the country, significant regional variation in the UK also occurs. There appears to be far more circulating virus in England than Scotland, reflected by anti-HEV IgG seroprevalence rates in blood donors of 12% and 4.6%, respectively [55,56] and congruently higher rates of viraemic blood donors in England [57]. Why there is such a regional variation within these countries is not known.
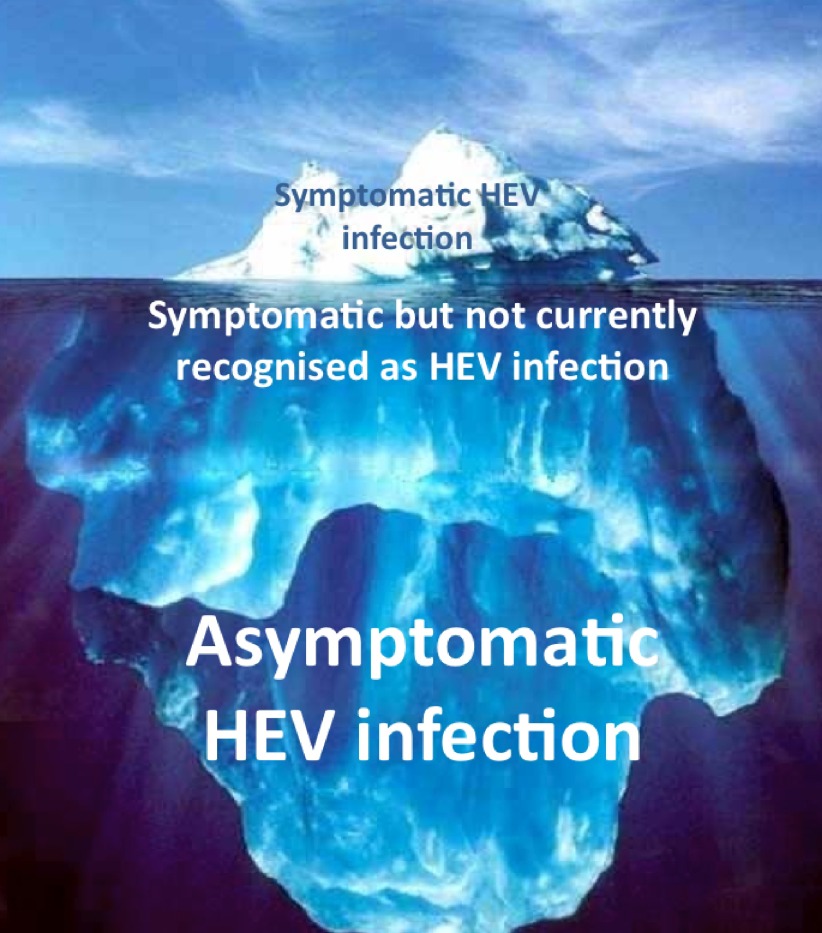
Each year it is estimated that in England there are 100,000 infections with HEV [57]. In 2013 there were just 691 laboratory confirmed cases [58], suggesting that most infections are either asymptomatic or unrecognised (Figure 4). In contrast with HEV genotype 1 where the ‘clinical attack rate’ is 50%, with zoonotic genotypes 3 and 4 as few as 5% of patients develop symptoms of acute hepatitis following exposure [1]. The incidence of unrecognised infection is unknown, and is the subject of ongoing study as the clinical phenotype of hepatitis E infection emerges (see below).
Figure 4.
The clinical spectrum of infection with HEV genotype 3. Most cases are asymptomatic; however, many are symptomatic, but not recognised
It has been known for some years that humans in developed countries can become re-infected with HEV genotype 1. Data from China suggest that 20% of infections with genotype 4 are re-infections, which appear to be more common in females and cause a milder hepatitis than primary infection [1]. Typically patients are IgM negative but IgG and PCR positive, so it can be difficult to establish a diagnosis. Until very recently in Europe, scant attention has been paid to the concept of re-infection. A recent study from Toulouse, France has shown that re-infections are surprisingly common in the transplant population [59]. In this setting, anti-HEV IgG antibody levels <7 WHO units/mL did not protect against re-infection. Now that we appreciate that there are large amounts of circulating HEV genotype 3 in Europe, the issue of re-infection merits further study.
HEV has been found in an increasingly diverse range of animals, most of which have no consequence for human infection [60,61]. HEV genotypes 3 and 4 have been found in pigs worldwide, and the pig is considered the primary host [6]. HEV has been found in all stages of the human food chain [62] and one established route of transmission from pigs to humans is via undercooked or uncooked pig meat products [63,64]. However, there are other possible ways this virus can be transmitted to humans; HEV has been found in soft fruits such as strawberries [65], watercourses [66] and the sea, and in shellfish [67]. It has also been found in the blood supply (see later). The relative importance of these other routes of transmission is not completely understood. Unlike HAV, household outbreaks have not been seen with zoonotic HEV. However, there have been reports of family members being asymptomatically infected, possibly from a common food source [14]. How frequently this occurs is unknown, and is the subject of ongoing study.
Places where HEV hides
It is now clear that autochthonous hepatitis E is extremely common in developed countries, where it has been described as an ‘emerging infection’ [6]. This is not strictly true, as HEV biological time-clock studies show that HEV diverged into its four genotypes several hundred years ago [68]. It would be more accurate to describe locally acquired hepatitis E in developed countries, as a disease that is ‘emerging in human consciousness’. How long has zoonotic HEV been causing human disease? No one knows for sure, but possibly hundreds of years [2]. During this time, HEV has very successfully evaded human scrutiny, due to its ability to ‘hide’ in diverse places.
Populations
Many early studies estimated the anti-HEV IgG seroprevalence in developed countries at <5% [69,70]. As a result, hepatitis E was thought not to be a health issue in these geographical settings. Some years ago we compared a commonly used commercial anti–HEV IgG assay (Genelabs) with an assay developed in China (Wantai), against a bank of acute and convalescent (up to 7 years post-infection) sera from PCR proven cases from southwest England [10]. The Chinese assay had a sensitivity of 98%, compared to 56% for the Genelabs assay. When applied to a population of blood donors the Genelabs assay underestimated the seroprevalence by a factor of four. The Chinese assay was subsequently applied to a population of blood donors in southwest France and the seroprevalence estimate increased from 16% to 52% with the more sensitive assay [71].
Sceptics have argued that a seroprevalence of 52% in Toulouse blood donors cannot possibly be correct, and that the result is due to lack of assay specificity. This is very unlikely to be true, as the seroprevalence in children aged 2–4 from the same population is low (2%) [71]. In addition, a seroprevalence of 52% is entirely congruent with the high incidence of primary and re–infections with hepatitis E documented in this community, both in transplant recipients [53] and asymptomatic blood donors [72]. These data suggest that much of the early literature is flawed, as assays of poor sensitivity have grossly underestimated the true seroprevalence, so enabling HEV to ‘hide’ at population level. More recent studies with the Chinese assay have shown much higher estimates than previously, with seroprevalence rates that are compatible with rates of HEV viraemia in asymptomatic blood donors (Table 4) [53,55–57,69–81].
Table 4.
HEV viraemia and seroprevalence in blood donors
| Country | Blood donors HEV RNA positive | HEV IgG seroprevalence (%) | Assay | Ref |
|---|---|---|---|---|
| SW France1 | 1:1595 | [72] | ||
| Midi–Pyrennes | 52.5 | Wantai | [71] | |
| 16 | Adaltis | [73] | ||
| France2 | 1:2218 | [72] | ||
| Germany | 1:1200 | [74] | ||
| 1:4525 | [75] | |||
| 29.5 | Wantai | [76] | ||
| 18.0 | Mikrogen | [76] | ||
| 4.5 | MP diagnosis | [76] | ||
| Netherlands | 1:2671 | 27.0 | Wantai | [53] |
| 1.1 | Abbott | [69] | ||
| England | 1:7000 | [77] | ||
| 12.0 | Wantai | [55] | ||
| 5.3* | Abbott | [70] | ||
| 1:2848 | [57] | |||
| Sweden | 1:7986 | [75] | ||
| 9.2* | Abbott | [78] | ||
| Scotland | 1:14520 | 4.7 | Wantai | [56] |
| USA | Nil | [75] | ||
| Nil2 | [79] | |||
| 16.0 | Wantai | [79] | ||
| Japan | 1:1781 | 3.7 | [80] | |
| China3 | 1:1493 | 32.6 | Wantai | [81] |
Data shown are from blood donors with the exception of those marked *, which are from healthy adults. HEV RNA was genotype 3 in all cases, except where stated.
Deconstructed solvent–detergent treated mini–pools.
Only 1,939 donors tested.
Of those with HEV viraemia, 57% were genotype1 and 43% genotype4.
Transplant recipients
Chronic hepatitis E infection in transplant recipients is clinically ‘silent’, as patients have no symptoms. The only clue to the diagnosis is a very modest elevation in serum ALT (100–300 IU/L) [1]. It is a diagnosis that is easily overlooked. How long has HEV been ‘hiding’ in transplant patients? This is unknown, but probably since the advent of transplantation in the late 1960s.
Drug-induced liver injury (DILI)
HEV can also masquerade as drug-induced liver injury (DILI). Some years ago we studied patients with criterion-referenced DILI and found that in six of 47 patients (13%) we had made a diagnostic error, as their illness was not due to DILI, but infection with HEV genotype 3 [18]. This is an easy diagnostic error to make, as both DILI and HEV genotype 3 infection are common in the elderly.
Neurological illness
Over recent years there have been quite a few case series and reports describing HEV-associated neurological illness. There appears to be a very wide spectrum of reported neurological injury that includes Bell's palsy, encephalitis, vestibular neuritis, small fibre peripheral neuropathy, Guillain–Barré syndrome and brachial neuritis [20,26]. In some cases HEV RNA has been found in the cerebrospinal fluid. The pathogenic mechanisms are unknown.
Guillain–Barré syndrome is a post infectious immune-mediated polyradiculopathy triggered by Campylobacter in 35% of cases but with an unknown aetiology in 50% of cases. In a prospective longitudinal study in 100 patients in the mid-1990s, the Dutch Guillain-Barré Study Group, found that 30% of patients had unexplained mildly abnormal liver function tests (LFT) at the start of the neurological illness [82]. A recent case control study of 201 patients with Guillain–Barré syndrome from the Netherlands showed that 5% of patients (n=10) had evidence of hepatitis E infection at the start of their neurological illness [83] and of these, three patients (1.5%) were viraemic with HEV genotype 3 at presentation. This has raised the question of whether these patients might benefit from early treatment with ribavirin therapy.
What about the other 25% of patients with Guillain–Barré syndrome and abnormal LFTs? Could these cases have also been triggered by HEV? One possible explanation could be that these cases might have been triggered re-infection with HEV. If the re–infection with HEV was 1 or 2 months before the neurological symptoms started, it would be very difficult to make a diagnosis. Such patients would be IgM negative (typical of re-infection), HEV PCR negative (the viraemic ‘window’ lasts only a few weeks), but IgG positive. Thus, the only way of distinguishing recent re–infection from distant past infection would be to demonstrate a rising IgG. This is problematic in this cohort of patients, as many are treated with intravenous immunoglobulin, which may well interfere with the IgG result on the convalescent blood sample.
A further Anglo-Dutch cohort study of 47 patients with brachial neuritis showed that 10% (n=5) had evidence of hepatitis E infection at the onset of neurological symptoms [84]. In contrast to other triggers of brachial neuritis, HEV-associated cases had bilateral neurological symptoms, sometimes with phrenic nerve involvement. Following this study, a brachial neuritis registry has been established in southwest England, with several further HEV–associated cases documented in just a few months (unpublished observations). A similar registry is being established in the Netherlands.
In both the above studies, neurological symptoms and signs dominated the clinical picture: patients were anicteric, the ALT was only mildly elevated (typically <600 IU/L), and occasionally normal. This led the lead neurologist to pose the following question: ‘Is it possible that hepatitis E has been misnamed? These patients have profound neurological illness, but not much of a hepatitis!’ This is an interesting question. The first step in the process of addressing this issue is about to start: a UK–Dutch–French multinational study of all patients with non–traumatic neurological injury who will be systematically tested for HEV at presentation. The results are awaited with interest.
C urrent diagnostic testing algorithms
In the UK, and in most of the rest of Europe, current diagnostic testing algorithms suggest that patients presenting with acute hepatitis should first be tested for HAV, HBV and HCV. If these tests are negative, then testing for HEV should be considered [85]. This approach is outdated, and means that the diagnosis of hepatitis E is either delayed, or missed altogether.
As hepatitis E is the commonest cause of acute viral hepatitis (Table 1, Figure 2) [24,25] , it would make much more sense to first test all patients for HEV, and if this is negative then consider testing for HAV, HBV and HCV. How should we define ‘hepatitis’? Preliminary data from southwest England suggests that testing patients with an ALT >400 IU/L or with an ALT/alkaline phosphatase ratio >6 times the upper limit of normal has quite high sensitivity and specificity for HEV diagnosis. Patients with Guillain–Barré syndrome and brachial neuritis should be tested for HEV irrespective of the ALT result. In addition, clinicians should have a low threshold for testing patients with unexplained neurological symptoms and abnormal LFTs. Immunosuppressed patients with persistently abnormal LFTs should be tested for HEV to exclude chronic infection. This should include PCR as well as serology, as the latter is less accurate in the immunosuppressed.
Blood supply
One of the potentially most worrisome places that HEV has been ‘hiding’ is in human blood products used for transfusion. Given that zoonotically acquired hepatitis E is very commonly asymptomatic, it comes as no surprise that HEV has found its way into the blood supply. What has astonished some observers is the very high incidence of HEV viraemia in the donor population (Table 4) [53,55–57,69–81]. Transmission of HEV via blood products is currently occurring as donors are not screened, and there are increasing numbers of reports of both acute and chronic infection in recipients (Table 5) [57,86–97].
Table 5.
Transfusion–transmitted HEV infection
| Country, Year | Ref | Number of infections / exposed | Comments |
|---|---|---|---|
| UK, 2014 | [57] | 18 / 43 | Retrospective study of 43 transfusion episodes available for investigation, 18 patients developed HEV |
| France, 2014 | [86] | 5 | Immunosuppressed liver transplant recipients. Outcome not detailed |
| France, 2014 | [87] | 2 | Immunosuppressed transplant recipients treated with plasmapheresis with Intercept-treated plasma. Both developed asymptomatic chronic infection requiring ribavirin therapy |
| Japan, 2014 | [88] | 1 | Immunosuppressed patient (myelodysplastic syndrome), received packed red cells from HEV-positive donor. Clinical hepatitis. Patient died from an unrelated cause (lung abscess) |
| Japan, 2014 | [89] | 1 | Immunocompetent patient, received HEV-contaminated platelet transfusion and developed post–transfusion acute hepatitis |
| France, 2013 | [90] | 1 | Immunosuppressed liver transplant recipient received HEV-contaminated packed red cell transfusion. Developed post-transfusion acute hepatitis. HEV cleared with ribavirin |
| Germany, 2013 | [91] | Six blood products identified from one donor. Retrospective study:
|
|
| France, 2012 | [92] | 1 | One immunosuppressed patient, on prednisolone then cyclosporine, developed clinical hepatitis. Cleared virus when immunosuppression stopped. Died from underlying condition |
| Japan, 2008 | [93] | 1 | Retrospective study. Platelet transfusion with HEV 4. Recipient developed clinical acute hepatitis |
| Japan, 2007 | [94] | 1 | Immunosuppressed patient with T-cell lymphoma on chemotherapy. Chronic infection after HEV–contaminated red cell transfusion |
| France, 2007 | [95] | 1 | 7-year-old immunosuppressed child, on chemotherapy. Acute post-transfusion hepatitis |
| UK, 2006 | [96] | 1 / 2 | Two patients received HEV-contaminated blood products. Immunocompetent recipient did not develop HEV. Second recipient was immunosuppressed (lymphoma on chemotherapy) and developed acute post-transfusion hepatitis |
| Japan, 2004 | [97] | 1 | Acute post-transfusion hepatitis following receipt of HEV-infected fresh frozen plasma |
In all the above studies, HEV was genotype 3 where sequencing data were available, unless otherwise stated.
In southeast England, a recent study demonstrated HEV RNA–positive plasma pools in 0.04% of donor samples, with one in 2,848 donors having HEV (genotype 3) viraemia at the time of donation [57]. Retrospective analysis showed that blood components were given to 60 patients, 43 of whom were available for follow–up: the overall transmission rate of HEV was 42%, and infection was significantly more common from high viral-load donations and plasma-based blood products, and less likely if the donor sample contained anti-HEV antibodies. ‘Classical’ post-transfusion hepatitis was uncommon in infected recipients, with only one in 18 developing clinically apparent post-transfusion hepatitis. This is not so different from the ‘clinical attack rate’ seen in individuals infected orofaecally. Seven of 10 immunocompromised patients infected were viraemic at 3 months following exposure, which is the working definition of chronic infection with HEV (see above). Two were treated with antiviral agents. There were four deaths, three of which were from unrelated causes. With a prevalence of one in 2848 in this study, a projection across England leads to an estimated 100,000 infections and a total of 1,200 HEV-contaminated transfusion events in the year of the study [57].
There is particular concern about the use of plasmapheresis, which uses pooled plasma, often from thousands of donors. Current treatment methods appear not to remove or inactivate HEV from pooled plasma, and plasmapheresis has been shown to transmit HEV to transplant recipients in France [87].
Should we screen blood donors for HEV?
There is currently a very lively debate about whether blood donors should be screened for HEV [98]. This has been brought to a head by the recent Lancet paper by Hewitt and colleagues discussed above [57]. The authors concluded that: ‘on a clinical basis alone, the resulting minimal burden of disease does not signal a pressing need for donation screening at this time’. This statement is however reminiscent of the initial reluctance to consider screening for HCV over 20 years ago [98]. Protagonists of screening would argue that, in common with any retrospective analysis, data collection in the Hewitt study was far from complete and there were no follow-up data at all on 17 HEV–infected recipients. Also, our understanding of the places HEV ‘hides’ is still developing and the clinical phenotype of hepatitis E is still emerging. Nucleic acid amplification testing will begin in Europe in 2015 of pooled plasma processed with solvent detergent [72]. The issue of safety of plasma-derived medicinal products is also under active review by the European Medicines Agency.
If blood donors should be screened for HEV, how should this be done? The answer to this is uncertain, as most viraemic donors have a normal ALT, and often have absent anti-HEV antibodies [72,75]. Nucleic acid amplification testing will probably be the technique of choice, but this remains to be determined.
Conclusions
Zoonotic HEV is a remarkably successful virus. It has several niches in animals, and appears in various ways in humans. It causes acute and chronic hepatitis. It causes a range of neurological injury. It has found its way into the blood supply. Like other successful RNA viruses, such as HIV and HCV, it has achieved this by stealth; by its ability to ‘hide’ in human populations and individual patients. The clinical phenotype of hepatitis E continues to emerge, and we are probably some way off a full understanding of the burden of disease in humans. Most members of the public have never heard of HEV. Should we knowingly expose them to this virus by blood transfusion? We think not. That would be like allowing the virus to win at the game of ‘hide and seek’. Zoonotically acquired HEV is, arguably, one of the most successful zoonotic viral infections in human history, and has required little assistance from us humans to achieve this.
References
- 1. Kamar N, Bendall R, Legrand-Abravanel F et al. . Hepatitis E. Lancet 2012; 379: 2477– 2488. [DOI] [PubMed] [Google Scholar]
- 2. Purcell RH, Emerson SU.. Hepatitis E: an emerging awareness of an old disease. J Hepatol 2008; 48: 494– 503. [DOI] [PubMed] [Google Scholar]
- 3. Navaneethan U, Al Mohajer M, Shata MT.. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int 2008; 28: 1190– 1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rein DB, Stevens GA, Theaker J et al. . The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012; 55: 988– 997. [DOI] [PubMed] [Google Scholar]
- 5. Labrique AB, Sikder SS, Krain LJ et al. . Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis 2012; 18: 1401– 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalton HR, Bendall R, Ijaz S, Banks M.. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis 2008; 8: 698– 709. [DOI] [PubMed] [Google Scholar]
- 7. Ijaz S, Arnold E, Banks M et al. . Non-travel-associated hepatitis E in England and Wales: demographic, clinical, and molecular epidemiological characteristics. J Infect Dis 2005; 192: 1166– 1172. [DOI] [PubMed] [Google Scholar]
- 8. Banks M, Grierson S, Fellows HJ et al. . Transmission of hepatitis E virus. Vet Rec 2007; 160: 202. [DOI] [PubMed] [Google Scholar]
- 9. Dalton HR, Thurairajah PH, Fellows HJ et al. . Autochthonous hepatitis E in southwest England. J Viral Hepat 2007; 14: 304– 309. [DOI] [PubMed] [Google Scholar]
- 10. Bendall R, Ellis V, Ijaz S et al. . A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol 2010; 82: 799– 805. [DOI] [PubMed] [Google Scholar]
- 11. Bendall R, Ellis V, Ijaz S et al. . Serological response to hepatitis E virus genotype 3 infection: IgG quantitation, avidity, and IgM response. J Med Virol 2008; 80: 95– 101. [DOI] [PubMed] [Google Scholar]
- 12. Dalton HR, Bendall RP, Keane FE et al. . Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med 2009; 361: 1025– 1027. [DOI] [PubMed] [Google Scholar]
- 13. Dalton HR, Stableforth W, Thurairajah P et al. . Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol 2008; 20: 784– 790. [DOI] [PubMed] [Google Scholar]
- 14. Dalton HR, Fellows HJ, Gane EJ et al. . Hepatitis E in New Zealand. J Gastroenterol Hepatol 2007; 22: 1236– 1240. [DOI] [PubMed] [Google Scholar]
- 15. Dalton HR, Stableforth W, Hazeldine S et al. . Autochthonous hepatitis E in Southwest England: a comparison with hepatitis A. Eur J Clin Microbiol Infect Dis 2008; 27: 579– 585. [DOI] [PubMed] [Google Scholar]
- 16. Lockwood GL, Fernandez-Barredo S, Bendall R et al. . Hepatitis E autochthonous infection in chronic liver disease. Eur J Gastroenterol Hepatol 2008; 20: 800– 803. [DOI] [PubMed] [Google Scholar]
- 17. Dalton HR, Bendall RP, Rashid M et al. . Host risk factors and autochthonous hepatitis E infection. Eur J Gastroenterol Hepatol 2011; 23: 1200– 1205. [DOI] [PubMed] [Google Scholar]
- 18. Dalton HR, Fellows HJ, Stableforth W et al. . The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther 2007; 26: 1429– 1435. [DOI] [PubMed] [Google Scholar]
- 19. Dalton HR, Hazeldine S, Banks M et al. . Locally acquired hepatitis E in chronic liver disease. Lancet 2007; 369: 1260. [DOI] [PubMed] [Google Scholar]
- 20. Kamar N, Bendall RP, Peron JM et al. . Hepatitis E virus and neurologic disorders. Emerg Infect Dis 2011; 17: 173– 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peron JM, Dalton H, Izopet J, Kamar N.. Acute autochthonous hepatitis E in western patients with underlying chronic liver disease: a role for ribavirin? J Hepatol 2011; 54: 1323– 1324; author reply 1324–1325. [DOI] [PubMed] [Google Scholar]
- 22. Mitchell J, Hussaini H, McGovern D et al. . The ‘jaundice hotline’ for the rapid assessment of patients with jaundice. BMJ 2002; 325: 213– 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panayi V, Froud OJ, Vine L et al. . The natural history of autoimmune hepatitis presenting with jaundice. Eur J Gastroenterol Hepatol 2014; 26: 640– 645. [DOI] [PubMed] [Google Scholar]
- 24. Donaghy L, Barry FJ, Hunter JG et al. . Clinical and laboratory features and natural history of seronegative hepatitis in a nontransplant centre. Eur J Gastroenterol Hepatol 2013; 25: 1159– 1164. [DOI] [PubMed] [Google Scholar]
- 25. Vine LJ, Shepherd K, Hunter JG et al. . Characteristics of Epstein-Barr virus hepatitis among patients with jaundice or acute hepatitis. Aliment Pharmacol Ther 2012; 36: 16– 21. [DOI] [PubMed] [Google Scholar]
- 26. Woolson KL, Forbes A, Vine L et al. . Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment Pharmacol Ther 2014. [DOI] [PubMed] [Google Scholar]
- 27. Colson P, Romanet P, Moal V et al. . Autochthonous infections with hepatitis E virus genotype 4, France. Emerg Infect Dis 2012; 18: 1361– 1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garbuglia AR, Scognamiglio P, Petrosillo N et al. . Hepatitis E virus genotype 4 outbreak, Italy, 2011. Emerg Infect Dis 2013; 19: 110– 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mansuy JM, Peron JM, Abravanel F et al. . Hepatitis E in the south west of France in individuals who have never visited an endemic area. J Med Virol 2004; 74: 419– 424. [DOI] [PubMed] [Google Scholar]
- 30. Wichmann O, Schimanski S, Koch J et al. . Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis 2008; 198: 1732– 1741. [DOI] [PubMed] [Google Scholar]
- 31. Tsang TH, Denison EK, Williams HV et al. . Acute hepatitis E infection acquired in California. Clin Infect Dis 2000; 30: 618– 619. [DOI] [PubMed] [Google Scholar]
- 32. Mitsui T, Tsukamoto Y, Hirose A et al. . Distinct changing profiles of hepatitis A and E virus infection among patients with acute hepatitis, patients on maintenance hemodialysis and healthy individuals in Japan. J Med Virol 2006; 78: 1015– 1024. [DOI] [PubMed] [Google Scholar]
- 33. Drobeniuc J, Greene-Montfort T, Le NT et al. . Laboratory-based surveillance for hepatitis E virus infection, United States, 2005–2012. Emerg Infect Dis 2013; 19: 218– 222; quiz 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fourquet E, Mansuy JM, Bureau C et al. . Severe thrombocytopenia associated with acute autochthonous hepatitis E. J Clin Virol 2010; 48: 73– 74. [DOI] [PubMed] [Google Scholar]
- 35. Deniel C, Coton T, Brardjanian S et al. . Acute pancreatitis: a rare complication of acute hepatitis E. J Clin Virol 2011; 51: 202– 204. [DOI] [PubMed] [Google Scholar]
- 36. Serratrice J, Disdier P, Colson P et al. . Acute polyarthritis revealing hepatitis E. Clin Rheumatol 2007; 26: 1973– 1975. [DOI] [PubMed] [Google Scholar]
- 37. Dumoulin FL, Liese H.. Acute hepatitis E virus infection and autoimmune thyroiditis: yet another trigger? BMJ Case Rep 2012; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andersson MI, Hughes J, Gordon FH et al. . Of pigs and pregnancy. Lancet 2008; 372: 1192. [DOI] [PubMed] [Google Scholar]
- 39. Dalton HR. Hepatitis: hepatitis E and decompensated chronic liver disease. Nat Rev Gastroenterol Hepatol 2012; 9: 430– 432. [DOI] [PubMed] [Google Scholar]
- 40. Peron JM, Bureau C, Poirson H et al. . Fulminant liver failure from acute autochthonous hepatitis E in France: description of seven patients with acute hepatitis E and encephalopathy. J Viral Hepat 2007; 14: 298– 303. [DOI] [PubMed] [Google Scholar]
- 41. Kumar Acharya S, Kumar Sharma P, Singh R et al. . Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol 2007; 46: 387– 394. [DOI] [PubMed] [Google Scholar]
- 42. Kamar N, Selves J, Mansuy JM et al. . Hepatitis E virus and chronic hepatitis in organ–transplant recipients. N Engl J Med 2008; 358: 811– 817. [DOI] [PubMed] [Google Scholar]
- 43. Gerolami R, Moal V, Colson P.. Chronic hepatitis E with cirrhosis in a kidney–transplant recipient. N Engl J Med 2008; 358: 859– 860. [DOI] [PubMed] [Google Scholar]
- 44. Kamar N, Garrouste C, Haagsma EB et al. . Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011; 140: 1481– 1489. [DOI] [PubMed] [Google Scholar]
- 45. Kamar N, Abravanel F, Selves J et al. . Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation 2010; 89: 353– 360. [DOI] [PubMed] [Google Scholar]
- 46. Pas SD, Man RA, Mulders C et al. . Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis 2012; 18: 869– 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pischke S, Stiefel P, Franz B et al. . Chronic hepatitis E in heart transplant recipients. Am J Transplant 2012; 12: 3128– 3133. [DOI] [PubMed] [Google Scholar]
- 48. Eijk AA, Pas SD, Cornelissen JJ, Man RA.. Hepatitis E virus infection in hematopoietic stem cell transplant recipients. Curr Opin Infect Dis 2014; 27: 309– 315. [DOI] [PubMed] [Google Scholar]
- 49. Naik A, Gupta N, Goel D et al. . Lack of evidence of hepatitis E virus infection among renal transplant recipients in a disease-endemic area. J Viral Hepat 2013; 20: e138– 140. [DOI] [PubMed] [Google Scholar]
- 50. Kamar N, Izopet J, Tripon S et al. . Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med 2014; 370: 1111– 1120. [DOI] [PubMed] [Google Scholar]
- 51. Zhu FC, Zhang J, Zhang XF et al. . Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo–controlled, phase 3 trial. Lancet 2010; 376: 895– 902. [DOI] [PubMed] [Google Scholar]
- 52. Faramawi MF, Johnson E, Chen S, Pannala PR.. The incidence of hepatitis E virus infection in the general population of the USA. Epidemiol Infect 2011; 139: 1145– 1150. [DOI] [PubMed] [Google Scholar]
- 53. Slot E, Hogema BM, Riezebos-Brilman A et al. . Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill 2013; 18. [DOI] [PubMed] [Google Scholar]
- 54. Legrand-Abravanel F, Kamar N, Sandres-Saune K et al. . Hepatitis E virus infection without reactivation in solid-organ transplant recipients, France. Emerg Infect Dis 2011; 17: 30– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Beale MA, Tettmar K, Szypulska R et al. . Is there evidence of recent hepatitis E virus infection in English and North Welsh blood donors? Vox Sang 2011; 100: 340– 342. [DOI] [PubMed] [Google Scholar]
- 56. Cleland A, Smith L, Crossan C et al. . Hepatitis E virus in Scottish blood donors. Vox Sang 2013; 105: 283– 289. [DOI] [PubMed] [Google Scholar]
- 57. Hewitt PE, Ijaz S, Brailsford SR et al. . Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet 2014. [DOI] [PubMed] [Google Scholar]
- 58. Public Health England Hepatitis E: Surveillance. The National Archives; 2014. Available from: http://webarchive.nationalarchives.gov.uk/20140714084352/ http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/HepatitisE/Surveillance/
- 59. Abravanel F, Lhomme S, Chapuy-Regaud S et al. . Hepatitis E virus reinfections in solid-organ-transplant recipients can evolve into chronic infections. J Infect Dis 2014; 209: 1900– 1906. [DOI] [PubMed] [Google Scholar]
- 60. Izopet J, Dubois M, Bertagnoli S et al. . Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg Infect Dis 2012; 18: 1274– 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Purcell RH, Engle RE, Rood MP et al. . Hepatitis E virus in rats, Los Angeles, California, USA. Emerg Infect Dis 2011; 17: 2216– 2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Berto A, Martelli F, Grierson S, Banks M.. Hepatitis E virus in pork food chain, United Kingdom, 2009–2010. Emerg Infect Dis 2012; 18: 1358– 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Colson P, Borentain P, Queyriaux B et al. . Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis 2010; 202: 825– 834. [DOI] [PubMed] [Google Scholar]
- 64. Pavio N, Merbah T, Thebault A.. Frequent hepatitis E virus contamination in food containing raw pork liver, France. Emerg Infect Dis 2014; 20: 1925– 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brassard J, Gagne MJ, Genereux M, Cote C.. Detection of human food-borne and zoonotic viruses on irrigated, field-grown strawberries. Appl Environ Microbiol 2012; 78: 3763– 3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ishida S, Yoshizumi S, Ikeda T et al. . Detection and molecular characterization of hepatitis E virus in clinical, environmental and putative animal sources. Arch Virol 2012; 157: 2363– 2368. [DOI] [PubMed] [Google Scholar]
- 67. Crossan C, Baker PJ, Craft J et al. . Hepatitis E virus genotype 3 in shellfish, United Kingdom. Emerg Infect Dis 2012; 18: 2085– 2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Purdy MA, Khudyakov YE.. Evolutionary history and population dynamics of hepatitis E virus. PLoS One 2010; 5: e14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zaaijer HL, Kok M, Lelie PN et al. . Hepatitis E in The Netherlands: imported and endemic. Lancet 1993; 341: 826. [DOI] [PubMed] [Google Scholar]
- 70. Bernal W, Smith HM, Williams R.. A community prevalence study of antibodies to hepatitis A and E in inner-city London. J Med Virol 1996; 49: 230– 234. [DOI] [PubMed] [Google Scholar]
- 71. Mansuy JM, Bendall R, Legrand-Abravanel F et al. . Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis 2011; 17: 2309– 2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gallian P, L’homme S, Piquet Y et al. . Hepatitis E virus infections in blood donors, France. Emerg Infect Dis 2014; 20: 1914– 1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mansuy JM, Legrand-Abravanel F, Calot JP et al. . High prevalence of anti-hepatitis E virus antibodies in blood donors from South West France. J Med Virol 2008; 80: 289– 293. [DOI] [PubMed] [Google Scholar]
- 74. Vollmer T, Diekmann J, Johne R et al. . Novel approach for detection of hepatitis E virus infection in German blood donors. J Clin Microbiol 2012; 50: 2708– 2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baylis SA, Gartner T, Nick S et al. . Occurrence of hepatitis E virus RNA in plasma donations from Sweden, Germany and the United States. Vox Sang 2012; 103: 89– 90. [DOI] [PubMed] [Google Scholar]
- 76. Wenzel JJ, Preiss J, Schemmerer M et al. . Test performance characteristics of Anti–HEV IgG assays strongly influence hepatitis E seroprevalence estimates. J Infect Dis 2013; 207: 497– 500. [DOI] [PubMed] [Google Scholar]
- 77. Ijaz S, Szypulska R, Tettmar KI et al. . Detection of hepatitis E virus RNA in plasma mini-pools from blood donors in England. Vox Sang 2012; 102: 272. [DOI] [PubMed] [Google Scholar]
- 78. Olsen B, Axelsson-Olsson D, Thelin A, Weiland O.. Unexpected high prevalence of IgG-antibodies to hepatitis E virus in Swedish pig farmers and controls. Scand J Infect Dis 2006; 38: 55– 58. [DOI] [PubMed] [Google Scholar]
- 79. Xu C, Wang RY, Schechterly CA et al. . An assessment of hepatitis E virus (HEV) in US blood donors and recipients: no detectable HEV RNA in 1939 donors tested and no evidence for HEV transmission to 362 prospectively followed recipients. Transfusion 2013; 53: 2505– 2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fukuda S, Sunaga J, Saito N et al. . Prevalence of antibodies to hepatitis E virus among Japanese blood donors: identification of three blood donors infected with a genotype 3 hepatitis E virus. J Med Virol 2004; 73: 554– 561. [DOI] [PubMed] [Google Scholar]
- 81. Guo QS, Yan Q, Xiong JH et al. . Prevalence of hepatitis E virus in Chinese blood donors. J Clin Microbiol 2010; 48: 317– 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Oomes PG, Meche FG, Kleyweg RP.. Liver function disturbances in Guillain–Barré syndrome: a prospective longitudinal study in 100 patients. Dutch Guillain-Barré Study Group. Neurology 1996; 46: 96– 100. [DOI] [PubMed] [Google Scholar]
- 83. Berg B, Eijk AA, Pas SD et al. . Guillain-Barre syndrome associated with preceding hepatitis E virus infection. Neurology 2014; 82: 491– 497. [DOI] [PubMed] [Google Scholar]
- 84. Eijk JJ, Madden RG, Eijk AA et al. . Neuralgic amyotrophy and hepatitis E virus infection. Neurology 2014; 82: 498– 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Public Health England UK Standards for Microbiology Investigations: Investigation of Hepatitis. Clinical Guidance G5. In: Standards Unit MS, ed, 2014.
- 86. Feray C, Pawlotsky JM, Roque-Afonso AM et al. . Should we screen blood products for hepatitis E virus RNA? Lancet 2014; 383: 218. [DOI] [PubMed] [Google Scholar]
- 87. Hauser L, Roque-Afonso AM, Beyloune A et al. . Hepatitis E transmission by transfusion of Intercept blood system-treated plasma. Blood 2014; 123: 796– 797. [DOI] [PubMed] [Google Scholar]
- 88. Kimura Y, Gotoh A, Katagiri S et al. . Transfusion-transmitted hepatitis E in a patient with myelodysplastic syndromes. Blood Transfus 2014; 12: 103– 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Matsui T, Kang JH, Matsubayashi K et al. . Rare case of transfusion-transmitted hepatitis E from the blood of a donor infected with the hepatitis E virus genotype 3 indigenous to Japan: Viral dynamics from onset to recovery. Hepatol Res 2014. [DOI] [PubMed] [Google Scholar]
- 90. Coilly A, Haim-Boukobza S, Roche B et al. . Posttransplantation hepatitis E: transfusion-transmitted hepatitis rising from the ashes. Transplantation 2013; 96: e4– 6. [DOI] [PubMed] [Google Scholar]
- 91. Huzly D, Umhau M, Bettinger D et al. . Transfusion-transmitted hepatitis E in Germany, 2013. Euro Surveill 2014; 19. [DOI] [PubMed] [Google Scholar]
- 92. Haim-Boukobza S, Ferey MP, Vetillard AL et al. . Transfusion-transmitted hepatitis E in a misleading context of autoimmunity and drug-induced toxicity. J Hepatol 2012; 57: 1374– 1378. [DOI] [PubMed] [Google Scholar]
- 93. Matsubayashi K, Kang JH, Sakata H et al. . A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion 2008; 48: 1368– 1375. [DOI] [PubMed] [Google Scholar]
- 94. Tamura A, Shimizu YK, Tanaka T et al. . Persistent infection of hepatitis E virus transmitted by blood transfusion in a patient with T-cell lymphoma. Hepatol Res 2007; 37: 113– 120. [DOI] [PubMed] [Google Scholar]
- 95. Colson P, Coze C, Gallian P et al. . Transfusion-associated hepatitis E, France. Emerg Infect Dis 2007; 13: 648– 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Boxall E, Herborn A, Kochethu G et al. . Transfusion-transmitted hepatitis E in a ‘nonhyperendemic’ country. Transfus Med 2006; 16: 79– 83. [DOI] [PubMed] [Google Scholar]
- 97. Matsubayashi K, Nagaoka Y, Sakata H et al. . Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion 2004; 44: 934– 940. [DOI] [PubMed] [Google Scholar]
- 98. Pawlotsky JM. Hepatitis E screening for blood donations: an urgent need? Lancet 2014. [DOI] [PubMed] [Google Scholar]