Abstract
The limitations to establishing a viral reservoir facilitated by early cART in children could play a critical role in achieving natural control of viral replication upon discontinuation of cART, which could be defined as ‘functional cure’. Viral reservoirs could provide a persistent source of recrudescent viraemia after withdrawal of cART, despite temporary remission of HIV-1 infection, as observed in the ‘Mississippi baby’. Intensification of cART has been proposed as a strategy to control residual replication and to diminish the reservoirs. The effects of cART intensification with maraviroc persisted after discontinuation of the drug in HIV-1-infected adults. However, in HIV-1-infected children, the emergence of CXCR4-using variants occurs very early, and the use of CCR5 antagonists in these children as intensification therapy may not be the best alternative. New treatments to eradicate HIV-1 are focused on the activation of viral production from latently infected cells to purge and clear HIV-1 reservoirs. This strategy involves the use of a wide range of small molecules called latency-reversing agents (LRAs). Histone deacetylase inhibitors (HDACi) such as givinostat, belinostat and panobinostat, and class I-selective HDACis that include oxamflatin, NCH-51 and romidepsin, are the most advanced in clinical testing for HIV-1 LRAs. Panobinostat and romidepsin show an efficient reactivation profile in J89GFP cells, a lymphocyte HIV-1 latently infected cell line considered a relevant model to study post-integration HIV-1 latency and reactivation. Clinical trials with panobinostat and romidepsin have been performed in children with other pathologies and it could be reasonable to design a clinical trial using these drugs in combination with cART in HIV-1-infected children.
Keywords: vertically acquired HIV-1 infection, HIV-reactivation, HIV-latency, panobinostat, romidepsin
Introduction
Combination antiretroviral therapy (cART) has raised the life expectancy, reduced the incidence of opportunistic infections and improved the quality of life of HIV-1-infected individuals. AIDS-related mortality in children has decreased significantly with the wide availability of cART. During recent years, multiple studies have suggested the benefit of early administration of cART in every HIV-1-infected infant [1–4]. Therefore, international guidelines are now recommending initiation of cART in all HIV-1-infected infants aged less than one year regardless of clinical and immunological conditions ( http://whqlibdoc.who.int/publications/2010/9789241599801.eng.pdf; and http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf). HIV-1 infection is still a chronic infection with a great number of associated complications and cART needs to be administrated life-long [5]. Therefore, searching for an HIV-1 cure remains a priority. Two different forms of cure have been defined: (i) a ‘sterilising cure’, in which all replication-competent virus and infected cells are eliminated (such as in the ‘Berlin Patient’ [6]), and (ii) a ‘functional cure’, represented by ‘elite controllers’ who permanently control HIV-1 replication without cART [7] (such as in the ‘Mississippi baby case’). Both the Berlin patient and the Mississippi baby are exceptional; and both cases are completely different. HIV-1 infection has been eradicated in the Berlin patient, while the Mississippi baby maintained a low level of inactive latent virus, only detectable using sensitive droplet digital PCR [8]. Eradication in the Berlin patient was achieved after a complex medical process, while the Mississippi baby was the first case of a potential HIV-1 cure achieved using a only pharmacological cART. The reason for the success of this approach, which is known to be ineffective in adults, could rely on the particularities of the immune system that HIV-1 encounters in a fetus or a newborn. The main obstacle in achieving functional cure is the persistence of a viral reservoir, a pool of the HIV-1 genome integrated into long-living Tcells, and probably in other haematopoietic cells such as macrophages [9].
Although cART achieves undetectable plasma viral RNA and the normalisation of CD4 T cell levels in almost every patient, several studies have shown that HIV-1 remains incurable owing to the persistence of latently infected cells [10–12]. The majority of these cells are resting memory and naïve CD4 Tcells, and cells belonging to the monocyte/macrophage lineage that contain integrated provirus within their genome. These cells are the main force behind HIV-1 persistence under cART, which only impacts on actively replicating viruses and is therefore unable to eradicate the infection. For this reason, the most recent approaches to HIV cure are focused on the definition of new drug families that do not target the replication of HIV but rather the transcription of proviruses in CD4 T cells. In combination with cART, these drugs would make HIV-1 visible and harmless to the immune system. This may be achieved by implementing both pharmacological and immunological strategies to reactivate HIV-1 from latently infected cells. Nevertheless, reactivation may not be sufficient to eradicate the virus. Reinforcing HIV-1-specific immune responses and blocking potential new events of viral replication will probably help in reaching the final goal of eradication, or the alternative objective of a ‘functional cure’ for HIV-1 infection.
Persistence of the viral reservoir
In HIV-1-infected adults, the pool of latently infected resting CD4+ T cells has been the most intensely analysed HIV-1 reservoir, and is widely recognised as one of the major barriers to achieving eradication or ‘functional cure’ of HIV-1 infection [13–16]. First, the absence of consensus on stability of the viral reservoir caused a storm of controversy concerning the possibility that residual HIV-1 replication in subsets of CD4+ T cells in the lymphoid tissue may contribute to replenishment of the HIV-1 reservoir [17–21]. Secondly, HIV-1 infects CD4 T cells and requires some level of immune activation to replicate. HIV-1 infects mostly memory cells, in particular cells from gut-associated lymphoid tissue (GALT), which concentrates most of the activated CCR5-expressing memory CD4+ T cells. Various groups have drawn attention to GALT as a major HIV-1 reservoir in individuals receiving cART, since CD4+ T cell recovery is poorer in GALT, and viral replication remains higher with respect to peripheral blood. This persistent viral replication in GALT probably contributes to maintenance of the reservoir despite peripheral viral suppression [17,22–24]. GALT depletion is a major pathogenic event in HIV-1 infection and is associated with the establishment of a long-lived viral reservoir and disease progression in HIV-1-infected adults [25]. In contrast, in the immunological setting of a fetus, memory cells are virtually absent from the CD4 T cell compartment [26] and GALT is anatomically immature, since it requires commensal bacteria for full development [27]. In the absence of an optimal setting for replication, HIV-1 may be unable to establish a long-lived latent reservoir and therefore, under this particular situation, very early treatment could have an additional beneficial effect that cannot be observed in chronically HIV-1-infected adults.
HIV-infected children who initiate cART soon after birth do not display HIV-1-specific antibodies or cellular responses, thus indicating early control of viral replication [28,29]. Nevertheless, HIV-1 infection quickly establishes a viral reservoir, mainly in resting memory CD4+ Tcells. Although the memory Tcell population in peripheral blood is small in newborns [30], although ready to develop later in childhood [31], recent findings have shown the presence of HIV-1-susceptible memory CD4+ Tcells in the gut of newborns with predominantly Thelper- (Th) 1 and Th17 phenotype, highlighting that extensive adaptive immunity is present before birth and the gut mucosa is the preferential site for memory CD4+ T cells [32]. The limitations to establishing a viral reservoir facilitated by early cART in children could play a critical role in achieving natural control of viral replication upon discontinuation of cART, which could be defined as ‘functional cure’ [31,33–36]. On the other hand, viral reservoirs could provide a persistent source of recrudescent viraemia despite temporary remission of HIV-1 infection after withdrawal of cART [37], as observed in the Mississippi baby [8].
Intensification of effective cART
Intensification of effective cART has been proposed as a strategy to control residual replication and to diminish the HIV-1 reservoirs [38]. Despite some studies having failed to show any effect of ART intensification on the residual HIV-1 viraemia in patients with a history of chronic infection receiving cART [39], a study of 48 weeks' intensification with maraviroc has been associated with a trend towards a decrease in the size of the latent HIV-1 reservoir in memory Tcells in chronically HIV-1-infected patients on cART [40].
CCR5 receptor antagonists in the pipeline are of particular interest because of their mechanisms of action, which could provide a beneficial anti-inflammatory effect beyond their antiviral activity. This immunomodulation represents an added benefit because it could improve the treatment of HIV-associated chronic immunoactivation [25]. This condition increases the risk for serious non-AIDS-related illnesses, such as heart disease, metabolic complications, kidney problems and others. Interestingly, potentially important anti-inflammatory effects have been highlighted for cenicriviroc, which inhibits the CCR2 receptor regulating rapid monocyte mobilisation [41,42]. A body of evidence from Phase II/III clinical trials of investigational CCR5 antagonists (maraviroc and vicriviroc) in treatment-experienced HIV-1 adults indicates a 30 cells/μL [95% confidence interval (CI) 19–42] greater increase in CD4+ T cell count in individuals using CCR5 antagonists (maraviroc or vicriviroc) than in groups not using CCR5 antagonists, despite baseline plasma HIV-1 RNA and virological suppression. Robust immunological effects were also observed in antiretroviral-naïve subjects included in the MERIT clinical trial, when the group randomised to receive maraviroc showed greater increases in CD4+ T cell count than did those receiving efavirenz [43]. The efficacy of cART intensification with the addition of maraviroc has also been studied in individuals with incomplete CD4+ T cell recovery, due to the potential role of maraviroc on immunological recovery [43]. This intensification is clinically relevant mainly for subjects with low CD4+ T cell count, to avoid overall HIV-1-related mortality and morbidity [44–46]. While some researchers reported that 24 weeks of maraviroc intensification was not associated with a CD4+ T cell gain of at least 20 cells/μL [47], others researchers observed immunological benefits [48]. Although the mechanism of this effect is still unknown, the blockage of the CCR5 receptor with maraviroc was associated with an increase in circulating levels of CCR5 ligands [48–50]. Because these ligands may also signal through alternative chemokine receptors, such as CCR1, CCR3 and CCR4 [51–53], the maraviroc-mediated activation of immune cells through alternative chemokine receptors requires further investigation [48]. Interestingly, the results of two clinical trials showed the dynamics of the HIV-1 latent reservoir after discontinuation of the intensification of cART. The effects of cART intensification with maraviroc or raltegravir persisted at least 24 weeks after discontinuation of the drug [54,55]. However, it is very important to note the emergence of CXCR4-using HIV-1 variants in a minority of HIV-1-infected patients following treatment with the CCR5 antagonist maraviroc, which probably developed from a pre-treatment CXCR4-using viral reservoir [56]. Because, in vertically HIV-1-infected children, the emergence of CXCR4-using variants occurs very early [57], the use of CCR5 antagonists in these children as intensification therapy may not be the best alternative.
Latency-reversing agents
The establishment of long-lived latent HIV-1 reservoirs involves multiple processes and is mainly due to transcriptional gene silencing in resting memory CD4+ Tlymphocytes and other non-dividing cell types, including monocytes. New compounds targeting transcriptional repression have been recently proposed as pharmacological agents for purging latent HIV-1 from cellular reservoirs in individuals on cART. These pharmacological compounds should be coupled with very potent cART, which will prevent reactivated virus from infecting new host cells, while viral cytopathic effects and immune clearance will eliminate HIV-1-infected cells. Treatments for eradicating HIV-1 are focused on the activation of viral production from latently infected cells to purge and clear HIV-1 reservoirs. This strategy involves the use of a wide range of small molecules called latency-reversing agents (LRAs) [58]. These drugs include: (i) histone deacetylase inhibitors (HDACis) [59]; (ii) disulfiram, postulated to involve nuclear factor kB cells (NF-κB) [60,61]; (iii) the bromodomain-containing protein 4 (BRD4) inhibitor JQ1, which elicits effects through positive transcription of the elongation factor (P-TEFb) [62]; and (iv) protein kinase C (PKC) activators such as ingenols [63], prostratin [64], 1,2-diacylglycerol analogues [65] and bryostatin-1 [66–68]. The interest in these drugs has increased greatly and there are several clinical trials in progress investigating the safety and the effect of LRAs as disruptors of HIV latency. HDACis are the most advanced in clinical testing as HIV-1 anti-latency agents, due mainly to the synthesis in recent years of novel and more specific pan-HDACis such as givinostat, belinostat and panobinostat [69,70] and newly synthesised class I selective HDACis that include oxamflatin [71], NCH-51 [72] and romidepsin [73]. Recently published results from a clinical trial of the safety and the effect of panobinostat on HIV-1 expression in patients on suppressive cART postulated this compound as a promising reactivator of HIV-1 latency[70].
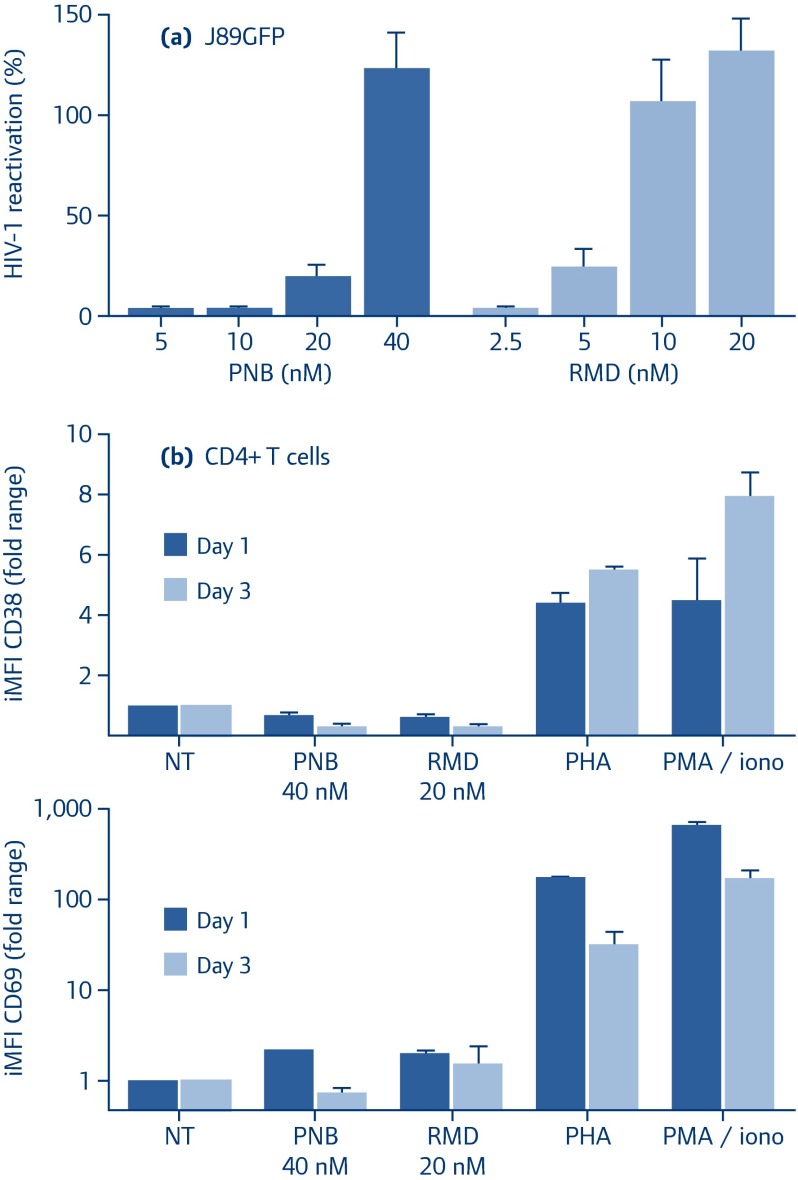
Both panobinostat and romidepsin show an efficient reactivation profile in J89GFP cells (Figure 1a), which is a lymphocyte HIV-1-latently infected cell line regarded as an experimentally tractable and relevant model to study post-integration HIV-1 latency and reactivation [74]. Moreover, the effects on primary CD4 Tcell activation, measured as the surface expression iMFI (integrated median fluorescence intensity) of CD38 and CD69 activation markers, has been assessed (Figure 1b). Although minimal effects in comparison with the conventional phytohaemagglutinin (PHA) or PMA/ionomycin treatments were observed after panobinostat or romidepsin exposure, a combinatorial strategy could lead to a reduction in the concentrations of LRAs used in vivo, resulting in a reduction of adverse effects, limiting the local injuries, the toxicity, and the inflammation.
Figure 1.
Reactivation effect of drugs alone or in double combinations.
(a) J89GFP cells were treated with PNB (dark coloured) and RMD (light coloured) at the indicated concentrations. After 24 hours, HIV-1 reactivation was analysed by flow cytometry as EGFP expression (iMFI). Percentage of HIV-1 reactivation was normalised to TNF-induced viral reactivation.
(b) Purified CD4+ T cells from healthy subjects were treated with the indicated concentrations of panobinostat and romidepsin or with PHA or PMA/ionomycin for 1 (dark bars) and 3 (light bars) days. The surface expression of the activation markers CD38 and CD69 in viable CD4+ T cells were analysed by flow cytometry and expressed as iMFI. Results represent the arithmetic mean+SEM of at least three independent experiments
EGPF: enhanced green fluorescent protein; iMFI: integrated median fluorescence intensity; PHA: phytohaemagglutinin; PMA/iono: PMA/ionomycin; PNB: panobinostat; RMD: romidepsin
To date, several clinical trials involving HIV-1-infected adults are ongoing with the aim of evaluating the safety, tolerability and the potency of these potential antiviral latency agents [75–77]. However, no data regarding potential anti-latency drugs in HIV-1 paediatric patients are available. Therefore, owing to the established differences between paediatric and the adult HIV infection, we cannot be certain about the impact of these drugs in HIV paediatric patients and whether they will help to establish a functional cure in HIV-1-infected paediatric patients. The effect of panobinostat has been studied in children ranging from age 8 to 21 years with refractory haematological malignancies ( https://clinicaltrials.gov/ct2/show/NCT01321346), and also in children older than 16 years with relapsing Hodgkin lymphoma ( https://clinicaltrials.gov/ct2/show/NCT01169636). On the other hand, romidepsin has been used in patients younger than 21 years with recurrent solid tumours or leukaemias ( https://clinicaltrials.gov/ct2/show/NCT00053963). These data suggest that it might be reasonable to design a clinical trial using these drugs in combination with cART in HIV-1 infected children and adolescents.
In summary, although there are still many obstacles before achieving a sterilising cure for HIV-1-infected paediatric patients, a functional cure could be close. Different approaches may be used to achieve it, although haematopoietic stem cell transplantation may not be used as a standard approach due to the elevated risks it carries. In recent infections, early cART may reduce the size of long-lived CD4+ T cell viral reservoirs that can be established, but the answers to several questions, such as the best cART and the optimum length of cART administration, remain elusive. Finally, in chronically HIV-infected paediatric patients, anti-latency drugs could have an important role but more information about the safety of these drugs in this population is required.
Conflicts of interest and funding sources
The authors declare that there are no conflicts of interest. This work has been (partially) funded by the RD12/0017/0037, project as part of the Plan Nacional R+D+I and co-financed by ISCIII- Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER), RETIC PT13/0010/0028, Fondo de Investigacion Sanitaria (FIS) (PI13/02016), Comunidad de Madrid (grant number S-2010/BMD-2332], PENTA, CYTED 214RT0482. CIBER-BBN is an initiative funded by the VI National R+D+i Plan 2008–2011, IniciativaIngenio 2010, the Consolider Program, and CIBER Actions and financed by the Instituto de Salud Carlos III with assistance from the European Regional Development Fund. MMB is supported by ‘Red de Investigación en SIDA’ (RIS).
References
- 1. Faye A, Le Chenadec J, Dollfus C et al. . Early versus deferred antiretroviral multidrug therapy in infants infected with HIV type 1. Clin Infect Dis 2004; 39: 1692– 1698. [DOI] [PubMed] [Google Scholar]
- 2. Luzuriaga K, McManus M, Mofenson L et al. . A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med 2004; 350: 2471– 2480. [DOI] [PubMed] [Google Scholar]
- 3. Violari A, Cotton MF, Gibb DM et al. . Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359: 2233– 2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prendergast A, Mphatswe W, Tudor-Williams G et al. . Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS 2008; 22: 1333– 1343. [DOI] [PubMed] [Google Scholar]
- 5. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382: 1525– 1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yukl SA, Boritz E, Busch M et al. . Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog 2013; 9: e1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 2012; 37: 377– 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ananworanich J, Robb ML. The transient HIV remission in the Mississippi baby: why is this good news? J Int AIDS Soc 2014; 17: 19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar A, Abbas W, Herbein G. HIV-1 Latency in Monocytes/Macrophages. Viruses 2014; 6: 1837– 1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Persaud D, Palumbo PE, Ziemniak C et al. . Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS 2012; 26: 1483– 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med 2002; 53: 557– 593. [DOI] [PubMed] [Google Scholar]
- 12. Siliciano JD, Kajdas J, Finzi D et al. . Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9: 727– 728. [DOI] [PubMed] [Google Scholar]
- 13. Brenchley JM, Hill BJ, Ambrozak DR et al. . T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol 2004; 78: 1160– 1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richman DD, Margolis DM, Delaney M et al. . The challenge of finding a cure for HIV infection. Science 2009; 323: 1304– 1307. [DOI] [PubMed] [Google Scholar]
- 15. Chomont N, El-Far M, Ancuta P et al. . HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15: 893– 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trono D, Van Lint C, Rouzioux C et al. . HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science 2010; 329: 174– 180. [DOI] [PubMed] [Google Scholar]
- 17. Chun TW, Nickle DC, Justement JS et al. . Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis 2008; 197: 714– 720. [DOI] [PubMed] [Google Scholar]
- 18. Kieffer TL, Finucane MM, Nettles RE et al. . Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J Infect Dis 2004; 189: 1452– 1465. [DOI] [PubMed] [Google Scholar]
- 19. Joos B, Fischer M, Kuster H et al. . HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci U S A 2008; 105: 16725– 16730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chun TW, Finzi D, Margolick J et al. . In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1995; 1: 1284– 1290. [DOI] [PubMed] [Google Scholar]
- 21. Chun TW, Fauci AS. HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. AIDS 2012; 26: 1261– 1268. [DOI] [PubMed] [Google Scholar]
- 22. Sheth PM, Chege D, Shin LY et al. . Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal Immunol 2008; 1: 382– 388. [DOI] [PubMed] [Google Scholar]
- 23. Yukl SA, Gianella S, Sinclair E et al. . Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis 2010; 202: 1553– 1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koelsch KK, Boesecke C, McBride K et al. . Impact of treatment with raltegravir during primary or chronic HIV infection on RNA decay characteristics and the HIV viral reservoir. AIDS 2011; 25: 2069– 2078. [DOI] [PubMed] [Google Scholar]
- 25. Brenchley JM, Price DA, Schacker TW et al. . Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12: 1365– 1371. [DOI] [PubMed] [Google Scholar]
- 26. Correa-Rocha R, Perez A, Lorente R et al. . Preterm neonates show marked leukopenia and lymphopenia that are associated with increased regulatory T-cell values and diminished IL-7. Pediatr Res 2012; 71: 590– 597. [DOI] [PubMed] [Google Scholar]
- 27. Rhee KJ, Sethupathi P, Driks A et al. . Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol 2004; 172: 1118– 1124. [DOI] [PubMed] [Google Scholar]
- 28. Persaud D, Ray SC, Kajdas J et al. . Slow human immunodeficiency virus type 1 evolution in viral reservoirs in infants treated with effective antiretroviral therapy. AIDS Res Hum Retrovirus 2007; 23: 381– 390. [DOI] [PubMed] [Google Scholar]
- 29. Persaud D, Siberry GK, Ahonkhai A et al. . Continued production of drug-sensitive human immunodeficiency virus type 1 in children on combination antiretroviral therapy who have undetectable viral loads. J Virol 2004; 78: 968– 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aldhous MC, Raab GM, Doherty KV et al. . Age-related ranges of memory, activation, and cytotoxic markers on CD4 and CD8 cells in children. J Clin Immunol 1994; 14: 289– 298. [DOI] [PubMed] [Google Scholar]
- 31. Shearer WT, Rosenblatt HM, Gelman RS et al. . Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol 2003; 112: 973– 980. [DOI] [PubMed] [Google Scholar]
- 32. Bunders MJ, Loos CM, Klarenbeek PL et al. . Memory CD4(+)CCR5(+) T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood 2012; 120: 4383– 4390. [DOI] [PubMed] [Google Scholar]
- 33. Saez-Cirion A, Bacchus C, Hocqueloux L et al. . Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9: e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Persaud D, Gay H, Ziemniak C et al. . Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369: 1828– 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ananworanich J, Schuetz A, Vandergeeten C et al. . Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7: e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Archin NM, Vaidya NK, Kuruc JD et al. . Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A 2012; 109: 9523– 9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klein N, Sefe D, Mosconi I et al. . The immunological and virological consequences of planned treatment interruptions in children with HIV infection. PloS One 2013; 8: e76582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chun TW, Justement JS, Moir S et al. . Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis 2007; 195: 1762– 1764. [DOI] [PubMed] [Google Scholar]
- 39. Dinoso JB, Kim SY, Wiegand AM et al. . Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A 2009; 106: 9403– 9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gutierrez C, Diaz L, Vallejo A et al. . Intensification of antiretroviral therapy with a CCR5 antagonist in patients with chronic HIV-1 infection: effect on T cells latently infected. PloS One 2011; 6: e27864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 2006; 7: 311– 317. [DOI] [PubMed] [Google Scholar]
- 42. Tsou CL, Peters W, Si Y et al. . Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 2007; 117: 902– 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cooper DA, Heera J, Goodrich J et al. . Maraviroc versus efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J Infect Dis 2010; 201: 803– 813. [DOI] [PubMed] [Google Scholar]
- 44. Lodwick RK, Sabin CA, Porter K et al. . Death rates in HIV-positive antiretroviral-naïve patients with CD4 count greater than 350 cells per microL in Europe and North America: a pooled cohort observational study. Lancet 2010; 376: 340– 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marin B, Thiebaut R, Bucher HC et al. . Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS 2009; 23: 1743– 1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bruyand M, Dabis F, Vandenhende MA et al. . HIV-induced immune deficiency is associated with a higher risk of hepatocarcinoma, ANRS CO3 Aquitaine Cohort, France, 1998–2008. J Hepatol 2011; 55: 1058– 1062. [DOI] [PubMed] [Google Scholar]
- 47. Wilkin TJ, Lalama CM, McKinnon J et al. . A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4(+) T-cell recovery despite sustained virologic suppression: ACTG A5256. J Infect Dis 2012; 206: 534– 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hunt PW, Shulman NS, Hayes TL et al. . The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood 2013; 121: 4635– 4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin YL, Mettling C, Portales P et al. . The chemokine CCL5 regulates the in vivo cell surface expression of its receptor, CCR5. AIDS 2008; 22: 430– 432. [DOI] [PubMed] [Google Scholar]
- 50. Nakata H, Kruhlak M, Kamata W et al. . Effects of CC chemokine receptor 5 (CCR5) inhibitors on the dynamics of CCR5 and CC-chemokine-CCR5 interactions. Antivir Ther 2010; 15: 321– 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guan E, Wang J, Roderiquez G, Norcross MA. Natural truncation of the chemokine MIP-1 beta /CCL4 affects receptor specificity but not anti-HIV-1 activity. J Biol Chem 2002; 277: 32348– 32352. [DOI] [PubMed] [Google Scholar]
- 52. Sarau HM, Rush JA, Foley JJ et al. . Characterization of functional chemokine receptors (CCR1 and CCR2) on EoL-3 cells: a model system to examine the role of chemokines in cell function. J Pharmacol Exp Ther 1997; 283: 411– 418. [PubMed] [Google Scholar]
- 53. Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat Med 1996; 2: 1174– 1178. [DOI] [PubMed] [Google Scholar]
- 54. Gutierrez C, Hernandez-Novoa B, Vallejo A et al. . Dynamics of the HIV-1 latent reservoir after discontinuation of the intensification of antiretroviral treatment: results of two clinical trials. AIDS 2013; 27: 2081– 2088. [DOI] [PubMed] [Google Scholar]
- 55. Vallejo A, Gutierrez C, Hernandez-Novoa B et al. . The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients. AIDS 2012; 26: 1885– 1894. [DOI] [PubMed] [Google Scholar]
- 56. Westby M, Lewis M, Whitcomb J et al. . Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV-1-infected patients following treatment with the CCR5 antagonist maraviroc is from a pretreatment CXCR4-using virus reservoir. J Virol 2006; 80: 4909– 4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Briz V, Garcia D, Mendez-Lagares G et al. . High prevalence of X4/DM-tropic variants in children and adolescents infected with HIV-1 by vertical transmission. Pediatr Infect Dis J 2012; 31: 1048– 1052. [DOI] [PubMed] [Google Scholar]
- 58. Kulkosky J, Bray S. HAART-persistent HIV-1 latent reservoirs: their origin, mechanisms of stability and potential strategies for eradication. Curr HIV Res 2006; 4: 199– 208. [DOI] [PubMed] [Google Scholar]
- 59. Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS 2011; 6: 25– 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xing S, Bullen CK, Shroff NS et al. . Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol 2011; 85: 6060– 6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Doyon G, Zerbato J, Mellors JW, Sluis-Cremer N. Disulfiram reactivates latent HIV-1 expression through depletion of the phosphatase and tensin homolog. AIDS 2013; 27: F7– F11. [DOI] [PubMed] [Google Scholar]
- 62. Zhu J, Gaiha GD, John SP et al. . Reactivation of latent HIV-1 by inhibition of BRD4. Cell Rep 2012; 2: 807– 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Warrilow D, Gardner J, Darnell GA et al. . HIV type 1 inhibition by protein kinase C modulatory compounds. AIDS Res Hum Retrovirus 2006; 22: 854– 864. [DOI] [PubMed] [Google Scholar]
- 64. Kulkosky J, Culnan DM, Roman J et al. . Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood 2001; 98: 3006– 3015. [DOI] [PubMed] [Google Scholar]
- 65. Hamer DH, Bocklandt S, McHugh L et al. . Rational design of drugs that induce human immunodeficiency virus replication. J Virol 2003; 77: 10227– 10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mehla R, Bivalkar-Mehla S, Zhang R et al. . Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PloS One 2010; 5: e11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Real G, Jimenez-Baranda S, Mira E et al. . Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med 2004; 200: 541– 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Perez M, Vinuesa AG, Sanchez-Duffhues G et al. . Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Curr HIV Res 2010; 8: 418– 429. [DOI] [PubMed] [Google Scholar]
- 69. Archin NM, Liberty AL, Kashuba AD et al. . Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012; 487: 482– 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rasmussen TA, Tolstrup M, Winckelmann A et al. . Eliminating the latent HIV reservoir by reactivation strategies: advancing to clinical trials. Hum Vaccin Immunother 2013; 9: 790– 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yin H, Zhang Y, Zhou X, Zhu H. Histonedeacetylase inhibitor Oxamflatin increase HIV-1 transcription by inducing histone modification in latently infected cells. Mol Biol Rep 2011; 38: 5071– 5078. [DOI] [PubMed] [Google Scholar]
- 72. Victoriano AF, Imai K, Togami H et al. . Novel histone deacetylase inhibitor NCH-51 activates latent HIV-1 gene expression. FEBS Lett 2011; l: 1103– 1111. [DOI] [PubMed] [Google Scholar]
- 73. Furumai R, Matsuyama A, Kobashi N et al. . FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res 2002; 62: 4916– 4921. [PubMed] [Google Scholar]
- 74. Kutsch O, Benveniste EN, Shaw GM, Levy DN. Direct and quantitative single-cell analysis of human immunodeficiency virus type 1 reactivation from latency. J Virol 2002; 76: 8776– 8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Archin NM, Sung JM, Garrido C, Soriano-Sarabia N, Margolis DM. Eradicating HIV-1 infection: seeking to clear a persistent pathogen. Nature Rev Microbiol 2014; 12: 750– 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Van Lint C, Bouchat S, Marcello A. HIV-1 transcription and latency: an update. Retrovirology 2013; 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Katlama C, Deeks SG, Autran B et al. . Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet 2013; 381: 2109– 2117. [DOI] [PMC free article] [PubMed] [Google Scholar]