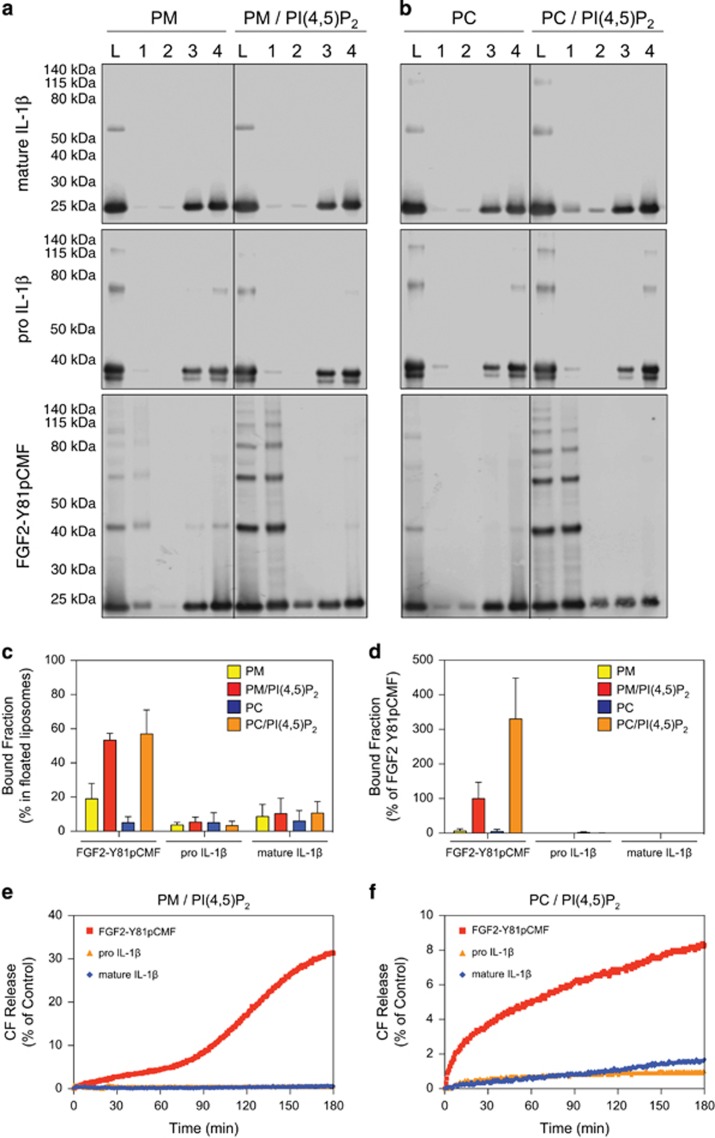
Figure 1.
Membrane binding and pore formation properties of pro-IL-1β, mature IL-1β, and phosphomimetic FGF2. (a–c) Biochemical analysis using liposomes characterised by either a plasma membrane-like lipid composition (PM) with or without 2 mol% PI(4,5)P2 (panel A) or phosphatidylcholine (PC) liposomes with or without 10 mol% PI(4,5)P2 (b). Binding properties of mature IL-1β, pro-IL-1β, and FGF2-Y81pCMF were compared. Membrane-bound material (fraction 1) was separated from free proteins (fractions 2–4) by membrane flotation in density gradients. Fractions were analysed by SDS-PAGE and western blotting using anti-IL-1β and anti-FGF2 antibodies, respectively. Quantification was done using an Odyssey infrared imaging system (LI-COR Bioscience). The amount of protein found in fraction 1 was quantified as the percentage of the total signal from fractions 1–4 (c). Mean values with S.D. of three independent experiments (n=3) are shown. (d) Binding of GFP-tagged variants of mature IL-1β, pro-IL-1β, and FGF2-Y81pCMF to liposomes with various lipid compositions as indicated was examined using an assay based upon flow cytometry. Signals were normalised based on FGF2-Y81pCMF binding to PM liposomes containing PI(4,5)P2 that was set to 100%. Mean values with S.D. (n=3) are shown. (e and f) Liposomes containing luminal carboxyfluorescein either consisting of a plasma membrane-like lipid composition (PM) containing PI(4,5)P2 (e) or phosphatidylcholine (PC) containing PI(4,5)P2 (f) were used to monitor membrane integrity upon incubation with the proteins indicated. Membrane pore formation was detected by the release of carboxyfluorescein that can be measured through dequenching. The results shown are representative for three independent experiments