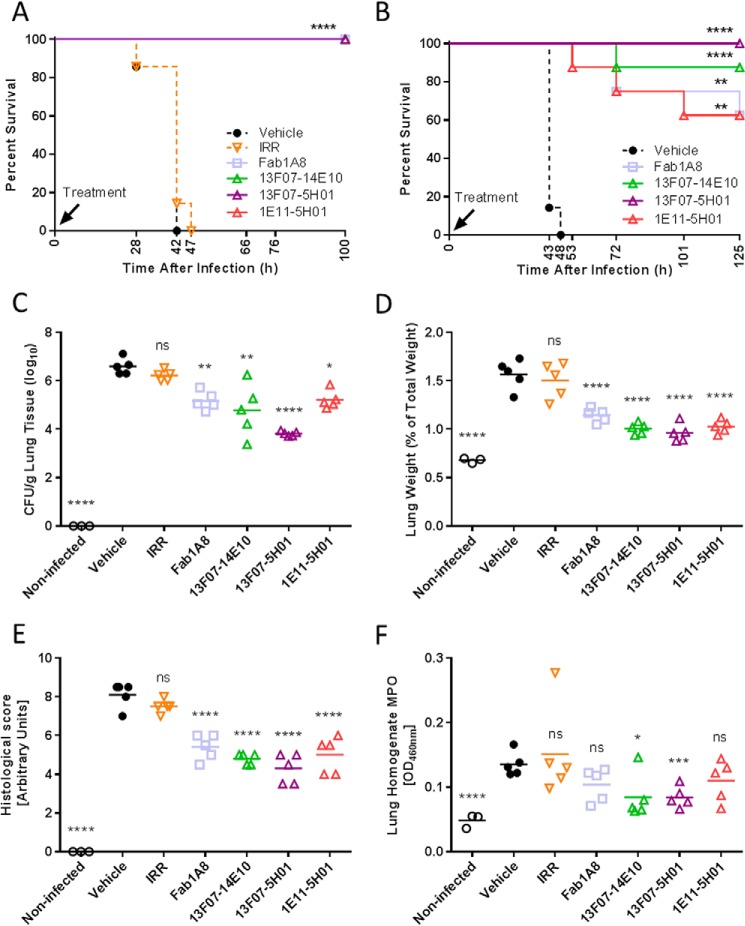
FIGURE 8.
In vivo characterization of biparatopic Nanobodies in a lethal acute P. aeruginosa-induced pneumonia model; prophylactic setting. A, survival comparison of C57BL/6 mice (7 mice/group) intranasally treated with a premix of anti-PcrV Nanobody (10 μg/mouse), anti-PcrV Fab1A8 (10 μg/mouse), irrelevant control Nanobody (IRR; 10 μg/mouse), or vehicle with 106 cfu of P. aeruginosa strain 2310.55 at the time of infection. B, survival comparison of C57BL/6 mice (7–8 mice/group) intranasally treated with a premix of anti-PcrV Nanobody (0.028 μg/mouse), anti-PcrV Fab1A8 (0.05 μg/mouse), or vehicle with 106 cfu of P. aeruginosa strain 2310.55 at the time of infection. Animals treated as in B (3–5 mice/group) were sacrificed at 24 h after P. aeruginosa challenge, after which the lung bacterial burden (C), lung weight relative to total body weight (D), lung histological score (E), and myeloperoxidase (MPO) activity (F) were determined. Each animal is represented as an open symbol, whereas the group means are shown as horizontal lines. IRR, an irrelevant control Nanobody (0.028 μg/mouse). Statistically significant differences between the experimental groups and the vehicle-treated group were determined using a log-rank test for the survival experiments or a linear model followed by a Hommel multiple testing correction (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, not significant).