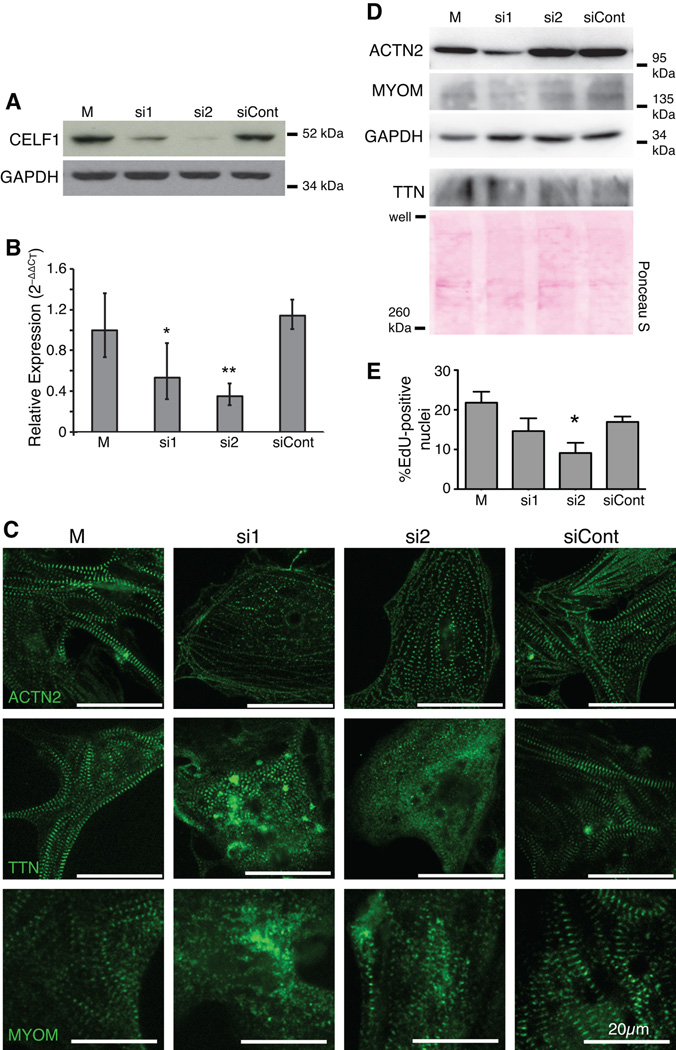
Fig. 2. Knockdown of CELF1 leads to disorganization of myofibrillar structure and reduced proliferation in primary embryonic cardiomyocytes.
(A) Depletion of CELF1 with two independent siRNAs (si1 and si2) but not a control siRNA (siCont) is seen by western blot at 72 hours post-transfection; M, mock transfection. Equivalent loading was confirmed by reprobing for GAPDH. (B) Transfection of cells with si1 and si2, but not siCont, resulted in robust knockdown of CELF1 transcripts as measured by real-time RT-PCR. Values shown are means ± 95% confidence intervals. *, p ≤ 0.05; **, p ≤ 0.01 versus mock-transfected cardiomyocytes as evaluated by Student’s t-test. (C) Knockdown of CELF1 at 24 hrs post-plating leads to disruption of myofibril organization as visualized by ACTN2, TTN, and MYOM immunofluorescence at 72 hrs post-transfection. (D) Western blots for ACTN2, TTN, and MYOM protein were performed on mock-, si1-, si2-, and siCont-transfected cardiomyocytes at 72 hrs post-transfection. Protein integrity, equivalent loading, and successful transfer were confirmed by reprobing for GAPDH or Ponceau S staining. (E) Proliferation was measured at 48 hrs post-transfection by EdU incorporation. Values shown are means + standard errors of the mean. *, p ≤ 0.05 versus siCont as evaluated by Student’s t-test.