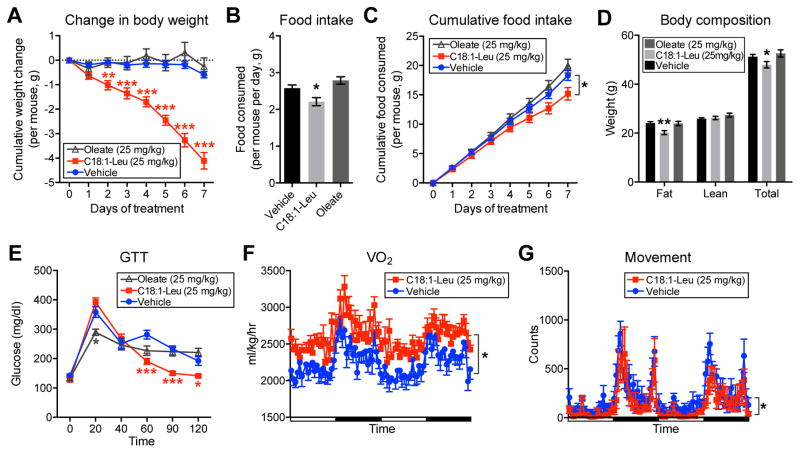
Figure 7. In vivo effects of chronic C18:1-Leu administration to mice.
(A–E) Change in body weight (A), daily and cumulative food intake (B and C), body composition by MRI (D), and GTT (E) of 21 week DIO mice treated daily with vehicle, or C18:1-Leu (25 mg/kg/day, i.p.), or oleate (25 mg/kg/day, i.p.). For (D), MRI measurements were taken on day 7. For (A–E), the initial weights of the mice were not statistically different (means ± SEM: vehicle, 51.9±0.8 g; 25 mg/kg C18:1-Leu, 52.1±1.1 g; 25 mg/kg oleate, 52.9±1.2 g). For (A–E), n=9/group, for vehicle and C18:1-Leu, and n=5/group for oleate, mean ± SEM, * p<0.05, ** p<0.01, *** p<0.001. For (E), after the last dose on day 7, mice were fasted overnight and the GTT was performed the next morning with glucose at a dose of 1.5 g/kg.
(F and G) VO2 (F) and movement (G) of mice treated with vehicle or C18:1-Leu. For (F and G), measurements were recorded for 2 days following 8 days chronic treatment with vehicle or C18:1-Leu (25 mg/kg/day, i.p.); during this time daily administration of the indicated compounds continued. For (F and G), n=8/group, mean ± SEM, * p<0.05. See also Figure S5, Figure S6, and Figure S7.