Abstract
Sertoli cells isolated from rodents or humans and cultured in vitro are known to establish a functional tight junction (TJ)-permeability barrier that mimics the blood-testis barrier (BTB) in vivo. This model has been widely used by investigators to study the biology of the TJ and the BTB. Studies have shown that environmental toxicants (e.g., perfluorooctanesulfonate (PFOS), bisphenol A (BPA) and cadmium) that exert their disruptive effects to induce Sertoli cell injury using this in vitro model are reproducible in studies in vivo. Thus, this in vitro system provides a convenient approach to probe the molecular mechanism(s) underlying toxicant-induced testis injury but also to provide new insights in understanding spermatogenesis, such as the biology of cell adhesion, spermatid transport, and others. Herein, we provide a brief and critical review based on studies using this in vitro model of Sertoli cell cultures using primary cells isolated from rodent testes versus humans to monitor environmental toxicant-mediated Sertoli cell injury, and this information is relevant to the molecular mechanisms that regulate spermatogenesis. In short, recent findings have shown that environmental toxicants exert their effects on Sertoli cells to induce testis injury through their action on Sertoli cell actin- and/or microtubule-based cytoskeleton. These effects are mediated via their disruptive effects on actin- and/or microtubule-binding proteins. Sertoli cells also utilize differential spatiotemporal expression of these actin binding proteins to confer plasticity to the BTB to regulate germ cell transport across the BTB.
Keywords: testis, spermatogenesis, Sertoli cells, toxicants, blood-testis barrier, ectoplasmic specialization, actin cytoskeleton
Introduction
Male infertility as indicated by declining semen quality, is on the rise among men. Studies in recent years have suggested the likely involvement of human exposure to environmental toxicants through the use of daily products, drinking water and foods, as well as smoking of cigarettes to be the cause [1, 2]. As such, there are needs to have a reliably in vitro system using testicular cells that can accurately assess the impact of environmental toxicants on male fertility. Furthermore, if such an in vitro model accurately predicts the outcome of reproductive health in male rodents and/or men in vivo following exposure to environmental toxicants, this model should theoretically be useful to unravel the molecular mechanism(s) by which toxicants exert their effects to disrupt male reproductive health and sheds insightful information in the regulation of spermatogenesis. Studies in the past decade have shown that the Sertoli cell is one of the primary targets of environment toxicants [3, 4] (Table 1). Studies have shown that primary cultures of Sertoli cells [5-18] as well as Sertoli cell line (e.g., SerW3) [19-21] in vitro provide a reliable model to study Sertoli cell blood-testis barrier (BTB) function and/or to monitor effects of xenobiotics on testicular function. More important, some of these findings have been reproduced in vivo [15, 22-24], illustrating Sertoli cells cultured in vitro indeed serve as a valuable and reliable model to study testicular function in particular BTB regulation. For instance, PFOS was recently shown to exert its disruptive effects to perturb the Sertoli cell tight junction (TJ)-permeability function via an activation of MAPK (mitogen activated protein kinase), including p38 and ERK, in vitro as well as in the testis in vivo [15]. Furthermore, the use of SB203580, a p38 MAPK selective inhibitor was capable of blocking the PFOS-induced Sertoli cell TJ-barrier disruption [15]. These findings are also in agreement with earlier reports that cadmium-induced BTB disruption is mediated by p38 MAPK through an up-regulation of TGF-β3 using the primary Sertoli cell in vitro model [12, 22]. More important, the use of SB202190, a p38 specific inhibitor, was found to block the cadmium-mediated Sertoli cell TJ-barrier in vitro and the BTB disruption in the testis in vivo [12, 22, 23]. Bisphenol A (BPA) was also shown to activate ERK1/2 in Sertoli cells cultured in vitro [25], consistent with findings that fetal exposure of rats to BPA also induced ERK1/2 mRNA expression [26]. Furthermore, treatment of neonatal rats (20-25 dpp, day postnatal) with BPA also induced Sertoli cell BTB disruption based on an in vivo functional assay [25]. Taken collectively, these findings thus support the notion that toxicants (e.g., cadmium, BPA, PFOS) perturb Sertoli cell BTB function is mediated by activating MAPK downstream, involving p38 and ERK1/2, and inhibitors that block the activation of these kinases can be possible candidates to alleviate toxicant-induced male infertility [27].
Table 1.
Effects of toxicants on Sertoli cell functions in rodents and humans*
| Species | Toxicant | Studies in vitro | Studies in vivo |
|---|---|---|---|
| Mouse Sertoli cells |
Mono (2- ethyhexyl) phthalate (MEHP) |
Oral gavage at 700 mg/kg/day for 3 consecutive days led to an increase TUNEL-positive cells, inducing germ cell degeneration [157] |
|
| Rat Sertoli cells |
2,5- Hexanedione (HD) |
0.08-1% HD in drinking water led to a dose-dependent increase in retaining spermatids in stages IX- XII tubules [144]; 40-211 mmol HD/kg b.w. induced Sertoli cell injury typified by cell vacuolation; also germ cell necrosis and exfoliation [158] |
|
| Bisphenol-A (BPA) |
Induced Sertoli cell apoptosis, up-regulation of Pten and Akt expression; reduced phopho-Akt and procaspase-3 expression [159]; BPA at 10 and 50 μM induced an increase in GSH level in Sertoli cells [18]; BPA at 200 μM perturbed Sertoli cell TJ- barrier function [25] |
BPA at 0.4 mg/kg b.w. impaired male fertility; neonatal rats exposed to BPA down-regulated Cx43 but up-regulated N-cadherin and ZO-1 expression [160]; BPA at 10-50 mg/kg b.w. induced small but insignificant increase in germ cell loss from seminiferous tubules in adult rats; BPA disrupted the BTB function in 20-day-old rats [25] |
|
| 1-dichloro-2,2 bis(p- chlorophenyl) ethylene (p,p’- DDE) |
Treatment of Sertoli cells with p’p- DDE (30 μM) for 24 hr induced cell apoptosis; up-regulation of Fasl mRNA and protein level; activation of caspase-3 and -8, as well as NF-κB [161, 162] |
||
| ß-Benzene | ß-Benzene (30 μM) induced Sertoli cell apoptosis via an increase in ROS [163] |
||
| 1,3- Dinitrobenzene (1,3-DNB) |
Rats treated with 1,3-DNB led to changes in the expression of genes that regulated apoptosis, cell junction integrity, and signaling pathways [164]; a single dose of 1,3-DNB at 25 mg/kg b.w. for 24 hr led to an increase in pachytene spermatocyte apoptosis, expression of Bax, Bcl-2, Bcl-xL, and Bcl-xs were up-regulated.[165]; daily administration of 1,3-DNB at 6 mg/kg b.w. led to reduced testis weight, loss of pachytene spermatocytes and Sertoli cell vacuolization in seminiferous tubules, and a reduced inhibin B expression [166] |
||
| Di-(2- ethylhexyl) phthalate (DEHP) |
Prepubertal rats treated with DEHP at 1 gm/kg b.w. by oral gavage reduction the expression of Cx43 and ZO-1, delaying the onset of spermatogenesis [167] |
||
| Cadmium | Treatment of Sertoli cells with CdCl2 (0.1-5 μM) perturbed the TJ-permeability barrier [11, 43] |
Treatment of adult rats with CdCl2
(30 μmol/kg b.w.; ~5 mg/kg b.w.) by i.p. induced irreversible disruption of the BTB, apoptosis and necrosis of testicular tissue [49, 50, 168]; adult rats treated with CdCl2 at 3 mg/kg b.w. by i.p. induced TGF-ß3 production that led to BTB disruption and p38 MAPK activation, which could be blocked by p38 specific inhibitor [22, 23]; CdCl2 also activated JNK to induce a2-macroglobulin to protect testes from unwanted proteolysis [51] |
|
| Di-n- pentylphthalate (DPP) |
Oral treatment of adult rats with DPP at 2200 mg/kg b.w. for 12 hr induced germ cell apoptosis in tubules in particular among differentiating spermatogonia and spermatocytes [169, 170] |
||
| 1,2-Dibromo-3- chloropropane (DBCP) |
Rats received DBCP once a week via s.c. for 3 weeks at 20 mg/kg b.w. led to reduced testis weight and infertility [171]; and an increase in serum gonadotropin level and intratesticular testosterone concentration [172] |
||
| Human Sertoli cells |
BPA | Human Sertoli cell treated with BPA at 20 or 200 μM for 2 days led to a decrease in the expression of ZO-1, N-cadherin, and ß-catenin; BPA also induced mis-localization of adhesion proteins at the cell-cell interface mediated via truncated and retracton of actin microfilaments from the Sertoli cell cortical zone; these changes in F-actin organization was mediated by an alteration in the localization of Eps8 and Arp3 [76] |
|
| CdCl2 | Treatment of human Sertoli cells with CdCl2 at 0.5-5 μM for 2 days induced re-distribution of ZO-1, N- cadherin, and ß-catenin with actin microfilaments re-localized by moving from the cell cortical zone to cell cytosol [76] |
||
| 1,2-Dibromo-3- chloropropane (DBCP) |
Severe impairment of spermatogenesis was found in 18 of 23 workers after exposure to DBCP [173] |
This Table is not intended to be exhaustive, only selected references are included to illustrate the effects of toxicants on rodent and human Sertoli cell function. Abbreviations used: Akt, murine thymoma viral oncogene homolog 1, also known as protein kinase B (PKB); Arp3, actin-related protein 3; Bax, Bcl2-associated X protein, also known as Bcl-2-like protein 4; Bcl, B-cell lymphoma; Bcl-xL, B-cell lymphoma-extra large; Bcl-xs, Bcl2-like 1; b.w., body weight; Cx43, connexin 43; Eps8, epidermal growth factor receptor pathway substrate 8; Fasl, Fas ligand, also known as apoptosis antigen 1; GSH, glutathione; i.p., intraperitoneal injection; JNK, c-Jun N-terminal protein kinase; NF-κB, nuclear factor kappa-light chain enhancer of activated B cells; p38 MAPK; p38 mitogen activated protein kinase; Pten, phosphatase and tensin homolog; ROS, reactive oxygen species; sc, subcutaneous injection; TJ, tight junction; ZO-1, zonula occludens 1.
In this context, it is of interest to note that studies conducted by toxicologists using rodents exposed to toxicants, such as carbendazim, phthalates, and 2,5-hexanedione have shown that one of the primary targets of toxicants in the testis is the Sertoli cell cytoskeletons, including microtubule (MT)-, actin-based and/or intermediate filament-based cytoskeletons (for reviews, see [28-32]). Yet the detailed molecular events and the underlying molecular mechanism(s) involving toxicant-mediated cytoskeletal disruption remain largely unknown in studies using whole animals, even though germ cell apoptosis [33, 34] and defects in fluid secretion into the seminiferous tubule [35] are the two emerging underlying mechanisms. This is due to the difficulty in discerning molecular changes, in particular the cascade of events and the involved molecules/proteins, using the testis as a whole. For instance, it would be difficult to visualize changes in actin and/or microtubule filament organization (e.g., defragmentation, retraction from Sertoli cell cortical zone) in Sertoli cells within the context of the seminiferous epithelium inside the testis in vivo. However, studies in vitro using Sertoli cells isolated from rats exposed to these toxicants have illustrated that 2,5-hexanedione indeed disrupts actin- [36], intermediate filament- [37] and MT- [35]. This thus supports the notion that 2,5-hexanedione impedes spermatogenesis and fertility in male rats is mediated through a disruption of Sertoli cell cytoskeletons. Recent studies have shown that toxicants exert their disruptive effects on F-actin network in the Sertoli cell through changes in the spatiotemporal expression of actin binding proteins (ABPs), stripping the ability of the actin microfilaments to re-organize themselves in response to changes of the epithelial cycle following exposure of Sertoli cells cultured in vitro to environmental toxicants (e.g., PFOS, cadmium, BPA) from both rodents and humans from multiple laboratories (Table 1). These findings support the notion that a loss of actin microfilament plasticity would impede Sertoli cell function, such as tight junction (TJ) permeability barrier.
Dosing issues of toxicants used for studies
Many earlier and recent studies were conducted using toxicants (e.g., cadmium, BPA, PFOS) at levels that were not close to those concentrations prevalent in the environment, nor following chronic exposures following much lower concentrations comparable to environmental exposure but similar to industrial or accidental exposure. However, some of these toxicants have a lengthy elimination half-life in humans, such as >20 years [38] and 5.4 years [39, 40] for cadmium and PFOS, respectively, vs. <2 hr for BPA [41, 42] (Table 2). Thus, a significant amount of cadmium and PFOS can be accumulated in human organs, such as the testis, over an extended period of time, similar to the concentrations used in these studies. There are few reports in the literature that assess the intratesticular level of toxicants following their acute exposure in adult rats. However, for in vitro studies in Sertoli cells, increasing concentrations, such as 0.1-10 μM for cadmium [11], 40-200 μM for BPA [25], and 5-20 μM for PFOS [14] were used in studies to monitor their effects on Sertoli cell TJ-barrier function. At these ranges, none of these toxicants were shown to be cytotoxic to Sertoli cells and a notable phenotype, such as a disruption of the TJ-barrier and changes in actin organization, was readily detectable, making them suitable for mechanistic studies. The concentration selected for subsequent mechanistic studies at 0.1 μM [43], 200 μM [25] and 20 μM [14] for cadmium, BPA and PFOS, respectively, are not cytotoxic to Sertoli cells [11, 14, 25]; in addition, these cells were capable of resealing their disrupted TJ-barrier following toxicant removal. Furthermore, the levels of these toxicants used for studies by our laboratory and other investigators were within the range used by investigators in earlier in vivo studies (see Tables 1 and 2). Thus, it is acceptable since these toxicants served as pharmacological probes to identify the targets of environment toxicants and to gain mechanistic insights regarding their likely mechanism(s) of action in the testis and to unravel the molecular mechanisms that regulate spermatogenesis.It is of interest to note that due to the fragile nature of primary Sertoli cells cultured in vitro, it is somewhat difficult to culture these cells over an extended period of time for chronic non-acute dose exposure. Additionally, studies using Sertoli cells isolated from adult rats following chronic exposed to 2,5-hexanedione that identified MT-based cytoskeleton to be one of the primary targets of toxicants are also consistent with findings in vivo as summarized above, illustrating the significance of this in vitro model. In fact, accumulating evidence has suggested that the Sertoli cell cytoskeleton is a common target of multiple toxicants (for reviews, see [29-31, 44-46]). Furthermore, other in vivo studies using cadmium chloride (CdCl2, Mr 183.31) also supported the notion that the Sertoli cell, such as the blood-testis barrier (BTB), is more susceptible to environmental toxicants. Earlier studies in which adult rats were treated with cadmium at ~3.7 mg/kg b.w. (0.02 mmol/kg b.w.) via a single subcutaneous injection caused extensive damage to the testis including germ cell exfoliation [47, 48]. At ~5 mg/kg b.w., it was found to induce severe testicular and vascular damage [49]. However, in a study that monitored the kinetics of Sertoli cell BTB vs. vascular damage following a single s.c. administration of CdCl2 at 5.5 mg/kg b.w. (30 μmol/kg b.w.) in adult, it was shown that the BTB was disrupted before vascular damage in the testis [50]. Using electron microscopy to monitor the integrity of the BTB vs. endothelial TJ integrity in microvessels in the interstitium in a kinetics study in which adult rats were treated with CdCl2 at 3 mg/kg b.w. via single administration via i.p. at 0 hr; the BTB was found to be disrupted by 10 hr, but endothelial TJ-barrier in microvessels was not damaged until 20 hr [51]. These findings were consistent with histological analysis since red blood cells were not detected in interstitial space until 20 hr, illustrating endothelial TJ-barrier disruption, but BTB was grossly affected by 14-16 hr based on fluorescence microscopy analysis [51]. Collectively, these findings support the notion that the BTB constituted exclusively by Sertoli cells in the testis is more susceptible to cadmium toxicity vs. the vascular TJ-barrier. In this context, it is of interest to note that other toxicants such as methoxyacetic acid (MAA) that disrupts pachytene spermatocytes and 1,3-dinitrobenzene (DNB) that targets Sertoli cells failed to perturb the BTB integrity, unlike cadmium [52], illustrating not all toxicants exerts their disruptive effects to induce testis injury through the same mechanistic pathway(s).
Table 2.
The acceptable daily intake/tolerable daily intake (ADI/TDI) dose and the half-life (t1/2) of common EDCs in humans
| Type of toxicant |
Toxicant | Human elimination half life |
Main Route (source) of exposure |
Estimated daily intake (per kg body weight) |
ADI or TDI (Adult) |
References |
|---|---|---|---|---|---|---|
| Heavy metal |
Cadmium | >20 years | Oral (seafood, rice, cigarette smoking) |
0.6-1 μg | 1 μg/kg/day | ATSDR,1999; [174, 175];www.popstoolkit.com/tools/HHRA/TDI_USEPA.aspx |
| Plasticizer | Bisphenol A | < 2 hr | Oral (food containers, water bottles) |
34 ng | 50 μg/kg/day | EFSA, 2006; [41, 42] |
| Phthalates (e.g., DEHP) |
10 hr | Oral, medical devices |
1.6 μg | 22 μ g/kg/day | USEPA, 1998; [176, 177] |
|
| Fungicide/ Pesticide |
Carbendazi m |
25 wk | Oral (daily utensils, food containers) |
1.5 μg | 0.02 mg/kg/day |
http://www.inchem.org/documents/jmpr/jmpmono/v95pr19.htm |
| Surfactant | PFOS | 5.4 years | Oral (carpets, upholstery, industrial products) |
1.6 ng | 150 ng/kg/day |
EFSA,2008; [39, 40]; http://www2.epa.gov/sites/production/files/2014-04/documents/factsheet_ contaminant_pfos_pfoa_ march2014.pdf |
| Solvent/cl e-aning agent (degrease r), major constituen t in gasoline |
2,5- Hexamedio ne (toxic metabolite of hexane) |
~13-14 hr | Inhalation | n.k. | 0.2 mg/kg/day |
http://www3.epa.gov/airtoxics/hlthef/hexane.html;
https://www.cdph.ca.gov/programs/hesis/Documents/nhexane_med_guide.pdf |
This Table is not intended to be exhaustive, it only contains several selected environmental toxicants that are discussed in this review, it provides the rationale of selecting the doses that were used in recent reports. Abbreviation used: ATSDR, Agency for Toxic Substances and Disease Registry; DEHP, di-2-ethylhexyl phthalate; EFSA, European Food Safety Authority; n.k., not known; PFOS, perfluorooctanesulfonic acid or perfluorooctane sulfonate; USEPA, US Environmental Protection Agency; **, WHO, World Health Organization; Oral, includes dairy products, meats, vegetables, fruits, seafood, beverage, and water.
We review some of these findings, and to provide a likely molecular pathway on toxicant-induced Sertoli cell injury. We caution that these findings must be further validated in better designed experiments in vivo. Additionally, more studies of this nature, in particular the use of human Sertoli cells in conjunction with rodent Sertoli cells, provide a reliable means to evaluate impacts of toxicants to humans. This information also provide insightful information on the biology of spermatogenesis.
Rat versus human Sertoli cell in vitro models to study environmental toxicant-mediated injury
As briefly discussed above, primary Sertoli cells isolated from 20-day-old male pups and cultured in vitro [53] is a widely used in vitro model to study toxicant-mediated Sertoli cell injury. Findings obtained from using this model also faithfully reproduce and/or reflect changes in the testis in vivo, such as the TJ-permeability barrier function, the apical tubulobulbar complex function, hormonal regulation of Sertoli-spermatid cell adhesion, Sertoli cell secretory function and Sertoli cell differentiation [6, 13, 15, 24, 54-57]. This in vitro model has also been used for mechanistic studies to probe the molecular events underlying toxicant-induced Sertoli cell TJ-barrier disruption or Sertoli cell-gonocyte interactions, such as cadmium, BPA, PFOS and phthalates, which faithfully reproduce findings in vivo such as by identifying the downstream signaling MAPKs p38, ERK1/2 or JNK (Table 1). Thus, Sertoli cells cultured in vitro is a good and reliable model to study male reproductive toxicology [58]. It must be noted that findings based on this in vitro system using rat (or mouse) Sertoli cells may not be applicable to reproductive toxicology in humans in vivo for a number of reasons. First, the metabolism of rodents is significantly faster vs. humans since daily food intake in rodents is equivalent to ~12-15% of their body weight [59]. Second, while humans and rodents share some similarity in spermatogenesis, these species are significantly different physiologically in many aspects [60-62]. For instance, each spermatogonium type Asingle in rodents vs. spermatogonium type Adark in humans produce up to 4096 vs. 16 haploid spermatids, respectively, illustrating higher spermatogenesis efficiency in rodents vs. humans [63, 64]. Third, rodents remain fertile when the level of sperm production is reduced by 90%, but this reduced sperm output leads to infertility in men, or at least subfertility [65]. Fourth, while toxicants are metabolized similarity in rodents and men in most cases, there are also differences [66]. For several xenobiotic metabolizing enzymes, such as the activity of aldehyde oxidase, glucuronosyltransferase and alcohol dehydrogenase, slight or no differences are found between humans and rodents, but the activity of flavin-dependent monooxigenase, glutathione-S-transferase and xanthine oxidase is higher in rodents than in humans, whereas the activity of microsomal epoxide hydrolase and methyl transferase is higher in human liver vs. rodents [67]. For cytochrome P450 (CYP), the most important drug-metabolizing enzyme family, while there are no large differences between humans and rodents regarding CYP2E1, there are considerable interspecies differences regarding catalytic activity for the species-specific isoforms of CYP1A, -2C, 2D and -3A [68]. Also, meperidine, a synthetic opioid, is rapidly hydrolyzed by dog, but not human liver microsomes [69]. PFOS (a perfluoralkyl sulfonate) and PFOA (perfluorooctanoic acid, a perfluoralkly carboxylate) are human-made perfluorinated chemicals (PFCs) that do not undergo biotransformation when ingested. However, PFOA is cleared more rapidly in urine and feces in rodents vs. humans [70, 71]. PFOA and PFOS have a biological half-life of ~4-5 years in humans [39, 70] (Table 2), illustrating that toxicokinetics of PFOA/PFOS in humans cannot be predicted based on rodent data. Therefore, findings obtained in rodents should be confirmed using human cells prior to clinical studies. Thus, it will be advantageous to set up a human Sertoli cell system in vitro which can be used in virtually any life science laboratory without sophisticated set up to monitor environmental toxicants before more resources are committed to confirm the health risks in a specific site or community. It is technically challenging to obtain fresh human testis samples for isolation of highly purified Sertoli cells versus rodents routinely in a research laboratory by taking into account of shipping costs, and other logistic/timing arrangements besides regulatory compliance and related paperwork. Technological advances in the field in recent years, however, have changed all this considerably. For instance, Sertoli cells isolated from ~17-20-day-old rat, or mouse testes, and cultured in serum-free chemically defined media such as F12/DMEM are differentiated and ceased to divide [72]. These Sertoli cells are also functionally similar to Sertoli cells isolated from adult rat testes [12, 73]. However, both mouse/rat and human Sertoli cells isolated from pups and adults are not terminally differentiated but mitotically active and proliferative when cultured in chemically defined media supplemented with fetal calf serum at ~5-10% [74, 75]. As such, human Sertoli cells obtained from several men at 12-, 23- and 36-years-old, who died following unexpected car accidents and/or illnesses are able to be maintained through ~4 to 5 passes in vitro, with about 8 to 50 days replication time to obtain 70-80% confluency [76]. Since the early 2010s, human Sertoli cells were commercially available through Lonza in the United States. In fact, our laboratory has used human Sertoli cells to probe the mechanism(s) by which cadmium and BPA perturb Sertoli cell function, in particular actin-based cytoskeleton [76].
In this context, it is of interest to note there are some studies in the literature in which investigators are using Sertoli cell line (e.g., SerW3 Sertoli cell line) to monitor the effects of toxicants on Sertoli cell function [19, 20, 77]. While the use of Sertoli cell line is convenient and less costly vs. primary Sertoli cell cultures, however, findings based on the use of a Sertoli cell line must be cautiously interpreted since some of these cells are immortal Sertoli cells. For instance, MSC-1 cells do not possess FSH receptors [78] and do not produce Mullerian inhibiting substance [79]; MSC-1 cells also do not secrete immunosuppressive biomolecules as of primary Sertoli cells, among other issues as noted by others, illustrating their usefulness to monitor toxicant-mediated testis injury (for a review, see [80]). Herein, we critically evaluate data obtained by exposing rat and human Sertoli cells to toxicants to provide insightful information on toxicant-mediated Sertoli cell dysfunction with emphasis on the likely mechanisms by which toxicants induced Sertoli cell BTB function through the disruption of actin-based cytoskeleton. As noted above, the information discussed herein is based mostly on studies using an acute dose of an environmental toxicant. While these earlier and recent studies used toxicants as pharmacological probes to investigate the functional machinery of Sertoli cells, these findings will need to be further evaluated and expanded in studies using rodent and/or human Sertoli cells subjected to chronic exposure at levels similar to the concentrations prevalent in the environment.
Sertoli cell cytoskeletons are one of the targets of environmental toxicants
Sertoli cells, similar to most motile cells in the mammalian body, are comprised of an extensive network of actin microfilaments known as stress fibers and also microtubules (MT) (Figure 1). When Sertoli cells are cultured in vitro they are motile cells, analogous to macrophages, neutrophils and metastatic cancer cells, capable of traversing the polyester (or nitrocellulose) membrane pores on Matrigel-coated bicameral units [81]. However, Sertoli cells in vivo are relatively static but highly polarized with their nuclei and intracellular organelles (e.g., phagosomes) located exclusively to the basal compartment [82, 83]. Since each Sertoli cell has to nurture ~30-50 germ cells at different stages of their development [84], such as by providing the lactate necessary to nourish germ cells [85, 86], it is not likely that Sertoli cells move across the seminiferous epithelium as when they are cultured in vitro. In order to coordinate cellular events during the epithelial cycle, Sertoli cells communicate with each other and also with germ cells to support cellular events in the epithelium via gap junctions and also with distant Sertoli cells across the seminiferous epithelium via intercellular bridges (also known as tunneling nanotubes), structures that are supported by both F-actin and MT [87-90]. Furthermore, developing germ cells in particular elongating spermatids must be transported across the seminiferous epithelium so that they can line-up near the luminal edge to prepare for spermiation at stage VIII of the epithelial cycle [62, 83, 91, 92]. Additionally, preleptotene spermatocytes transformed from type B spermatogonia residing in the basal compartment must also be transported across the immunological barrier at stage VIII of the epithelial cycle [62] so that meiosis I/II can take place in the adluminal compartment behind the BTB. These cellular events, including meiosis, also require the extensive involvement of both actin- and MT-based cytoskeletons. For instance, many of the TJ- and anchoring junction proteins, including gap junction and intercellular bridges, at the Sertoli-Sertoli and Sertoli-germ cell interface are using actin microfilaments for attachment, and germ cell transport requires the presence of polarized MT to serve as the track for their transport. Herein, we briefly summarize recent advances in the field regarding studies on actin- and MT-based cytoskeletons in Sertoli cells, and the mechanism by which environmental toxicants perturb these cytoskeletal ultrastructures.
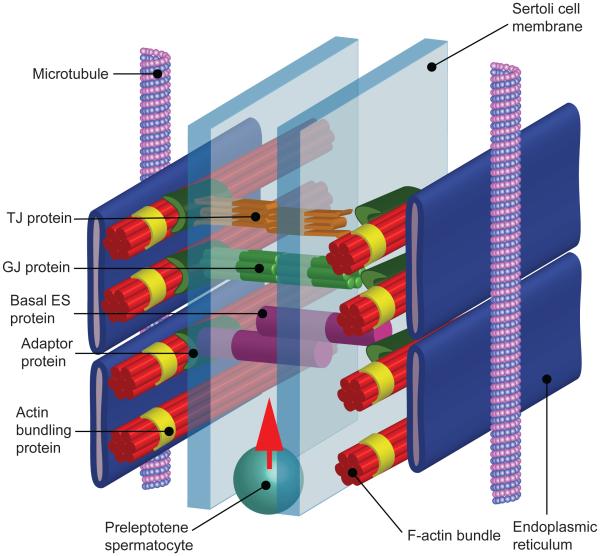
Figure 1. A schematic drawing illustrating the ulstrastructural features of actin-based adhesion proteins that constitute the blood-testis barrier (BTB) in rodents and humans.
The most noticeable structural feature of the BTB, constituted by tight junction (TJ), basal ectoplasmic specialization (basal ES), and gap junction (GJ) between adjacent Sertoli cells located near the basement membrane of the seminiferous epithelium, is the presence of an array of actin microfilament bundles that are sandwiched between cisternae of endoplasmic reticulum (ER) and apposing Sertoli cel plasma membranes. The BTB is located above the preleptotene spermatocytes which are set to be transported across the BTB at stage VIII of the epithelial cycle. As shown herein, adhesion protein complexes of the TJ (e.g., occludin-ZO-1), basal ES (e.g., N-cadherin-β-catenin), and GJ (e.g., connexin43-plakophilin-2) (note: occludin, N-cahderin, and connexin 43 are the integral membrane proteins of the TJ, basal ES and GJ, respectively, whereas ZO-1, β-catenin, and plakophilin-2 are the corresponding adaptor protein of TJ, basal ES and GJ) are anchored onto the actin microfilaments via the corresponding adpator protein. Actin microfilament bundles are maintained via the action of actin bundling proteins such as Eps8, pallain, and plastins. Microtubules (MT) also also located near the actin microfilaments which serve as the tracks for the transport of preleptotene spermatocytes across the BTB at stage VIII of the epithelial cycle.
F-actin-based cytoskeleton
Globular actin (G-actin, a 42 kDa polypeptide) with bound ATP can polymerize to form filamentous actin (F-actin) of different lengths [93-95]. F-actin is a linear polymer microfilament with intrinsic polarity such as the fast-growing end (i.e., barbed end) versus the slow-growing end (i.e., pointed end). In vertebrates, there are three isoforms of actin: α, β and γ. α−Actins are found in muscle cells and are a major constituent of the contractile apparatus, whereas β− and γ−actins coexist in mammalian cells, and are components of the cytoskeleton to modulate cell motility via actin-based ultrastructures known as filopodia (or microspikes) and lamellipodia, formed by actin filaments cross-linked into bundles by actin binding proteins (ABPs) such as fascins and plastins [93, 94, 96]. Eukaryotic cells, including Sertoli cells in the seminiferous epithelium, are equipped with different functional classes of ABPs to facilitate dynamic changes of actin microfilaments. These include ABPs that induce filament polymerization (i.e., nucleation) (e.g., formins), depolymerization/cleavage/severing (e.g., gelsolin, cofilin-1), barbed end capping (e.g., Eps8), bundling (e.g., Eps8, fascins, plastins, ezrin), cross-linking (e.g., α-actinin, filamin A), and branching (e.g., Arp2/3 complex, N-WASP) (Table 3). It is known that motor proteins such as myosin Va [97, 98], VI [99], VIIa [100] and IIB [101] specific to actin microfilaments serve as “vehicles” and work with polarized actin microfilaments that act as the “tracks” for spermatid transport [102, 103], and cytokinesis [101] for meiosis, as well as for intracellular transport of organelles through endocytic vesicle-mediated trafficking such as caveolae [104] for protein endocytosis and recycling, and proacrosomal vesicles [105] for acrosome assembly. These events are working in concert with MT [106-108], possibly through the involvement of actin binding proteins (ABPs), microtubule-associated proteins (MAPs), plus-end tracking proteins (+TIPs) and/or minus-end tracking proteins (−TIPs) [44, 109-113]. In the testis, actin microfilaments are highly concentrated in a testis-specific junction known as ectoplasmic specialization (ES) at the Sertoli cell-cell interface called basal ES at the BTB and also at the Sertoli-spermatid interface called apical ES [114-117]. At the ES, actin microfilaments that lie perpendicular to the Sertoli cell plasma membrane are bundled via the action of actin bundling proteins Eps8 (also a barbed-end capping protein) [118], palladin [119], plastin 3 [120], fascin 1 [121]. These arrays of actin filament bundles, in turn, are sandwiched between cisternae of endoplasmic reticulum and the apposing Sertoli cell-cell (basal ES) or Sertoli-spermatid (apical ES) to confer cell adhesion, cell polarity and germ cell transport (Figure 1).
Table 3.
Actin binding proteins (ABPs) in the testis *
| Function al type of actin binding proteins |
Actin bindin g protein s |
Localization in the seminiferous epithelium |
Interactin g partner proteins |
Phenotypes after KD or KO in vivo |
|---|---|---|---|---|
| Actin nucleatio n proteins |
Arp3 | At the apical ES, it was restricted to the concave side of spermatid heads at stage VII; at the basal ES/BTB, it was not expressed until stage VIII ES[178] |
Actin, members of the Arp2/3 complex, N-WASP |
Arp3 deficient mice died at blastocysts stage [179] |
| Formin 1 |
At the apical ES, it was restricted to the concave side of spermatid heads at stage VII; at the basal ES/BTB, it was expressed at stages I-VII [180] |
Actin, Arp3, α- tubulin- |
Formin 1 KO in mice led to a delay in bone formation [181], mice also had 4 digits, a deformed posterior metatarsal, phalangeal soft tissue fusion as well as the absence of a fibula [182]; KD of formin 1 impeded the transport of spermatid and phagosome due to the mis- organization of F-actin at the ES [180]. |
|
| Actin bundling/ cross- linking proteins |
Eps8 | At the apical ES, it was restricted to the concave side of spermatid heads at stage VII; at the basal ES/BTB, it was expressed at stages I-VII [118] |
Abi-1, IRSp53, Sos1 |
Eps8 KO mice were less sensitive and more tolerance to ethanol consumption [183]; these Eps8 KO mice appeared to be healthy and fertile [184]; microvilli in cells appeared disorganized and were significantly shorter versus the age-matched WT control mice [185]; Eps8 KD in the testis led to germ cell exfoliation and BTB disruption [118] |
| Ezrin | Ezrin was expressed at the basal ES/BTB in all stages of the epithelial cycle except at stage IX when its expression was considerably diminished [88]. |
Actin, Arp3, FAK, c- Src, JAM- A, laminin- γ3 chain, N- cadherin |
Ezrin KD in the testis impeded BTB integrity mediated by changes in the organization of F- actin at the ES [88]; ezrin KO led to death of pups before weaning [186]. |
|
| Fascin1 | At the apical ES, it was expressed at the concave side of step 19 spermatid heads; at the basal ES/BTB, it was expressed in all stages of the epithelial cycle except VIII [121] |
Actin, Arp3, β- catenin, nectin-3, occludin, palladin, Par6 |
Fascin 1 KO mice had deficient embryonic fibroblasts having shorter and less filopodia and KO mice had shorter life span, but they were viable and fertile [187]; fascin 1 KD in adult rat testes led to dis-organization of F- actin at the apical and basal ES, making the ES incapable of providing proper adhesion support to Sertoli and/or spermatid in which apical ES and TJ/basal ES adhesion proteins were mis-localized [121] |
|
| Palladi n |
Highly expressed at the apical ES in all stages, it also expressed at the basal ES/BTB in all stages but considerably down-regulated at VIII [119] |
Apr3, ARPC2, c- Src, Eps8 |
Palladin KO in mice led embryonic lethality due to neural tube closure defects [188]; palladin KD in the testis in vivo perturbed spermatid polarity, leading to defects in spermiation due to spermatid retention in the epithelium [119] |
|
| Plastin 3 |
At the apical ES, plastin 3 was detected at stages V-VII; at the basal ES/BTB, plastin 3 was found in all stages but considerably diminished at stage VIII [120] |
Actin, vimentin |
Plastin 3 variants in humans led to higher risk of bone fracture vs. noncarriers [189]; KD of plastin 3 in rat testes in vivo induced structural defects to the F-actin network, leading to premature spermatid release [120] |
This Table is not intended to be exhaustive, it is based on some recent findings illustrating the role of ABPs in spermatogenesis. Abbreviations used: Abi-1, Abelson interactor 1; Arp3, actin related protein 3; Arpc2, actin-related protein2/3 complex subunit 2; BTB, blood-testis barrier; c-Src, cellular-sarcoma proto-oncogene tyrosine-protein kinase; Eps8, epidermal growth factor receptor pathway substrate 8; ES, ectoplasmic specialization; FAK, focal adhesion kinase; JAM-A, junction adhesion molecule A; KD, knockdown; KO, knockout; N-WASP, neuronal Wiskott-Aldrich Syndrome protein; Par6, partitioning defective 6; TJ, tight junction.
Toxicants and Sertoli cell actin microfilaments
Interestingly, studies have shown that F-actin microfilaments are a prime target of toxicant-mediated Sertoli cell injury, including PFOS, BPA, cadmium, and adjudin [14, 45, 51, 76] (Figure 2). When human [76] or rat [14] Sertoli cells are cultured in vitro, distinctive actin microfilaments are detected that stretch across the cell cytosol including the cortical zone (Figure 2). Interestingly, exposure of human Sertoli cells to CdCl2 (5 to 20 μM) or BPA (40-200 μM) [76] or rat Sertoli cells to PFOS (20 μM) [14] (Figure 2) in vitro that had established a functional TJ-permeability barrier [14, 75] was found to induce rapid re-organization of actin microfilaments in these cells, making them disorganized, truncated and more significantly, causing actin filaments no longer neatly localized at the cortical zone to support Sertoli cell adhesion. Thus, TJ (e.g., occludin, ZO-1) and basal ES proteins (e.g., N-cadherin, β-catenin) could no longer localize at the cell-cell interface to support the TJ-barrier function since these adhesion protein complexes utilize actin cytoskeleton for attachment, thereby destabilizing the barrier function [14, 76]. While these are in vitro studies, these findings are consistent with earlier reports by using electron microscopy to show that treatment of adult rats with cadmium (CdCl2, 5 mg/kg b.w. via i.p.) [51] or adjudin (50 mg/kg b.w., via oral gavage) [122] also induced disorganization of actin microfilaments at the ES, including defragmentation of microfilaments.
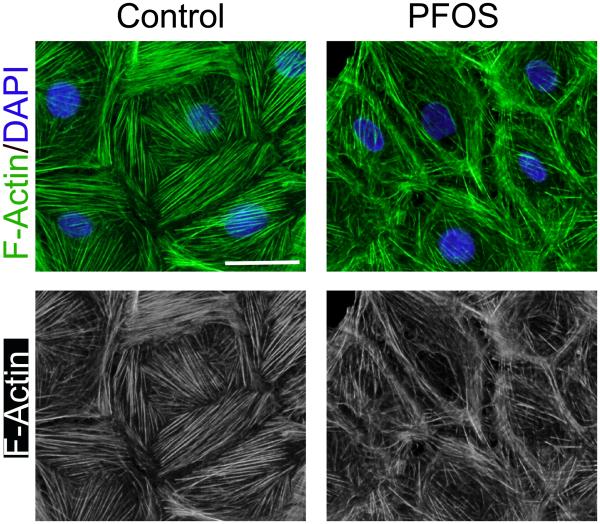
Figure 2. Actin microfilaments in rat Sertoli cells cultured in vitro and changes in their organization following treatment with PFOS.
Sertoli cell isolated from 20-day-old rat testes were cultured in serum-free F12/DMEM supplemented with growth factors for 4 days. Distinctive actin microfilaments (green fluurescence) that stretch across the Serotli cell are noted. However, following treatment of these Sertoli cells with PFOS at 20 μM for 24 hr, actin microfilaments are truncated. Thus, defragemented actin microfilaments no longer capable of supporting adhesion complexes, such as occludin-ZO-1 and N-cadherin-β-catenin, to confer cell adhesion, thereby destablizing the Sertoli cell FJ-permeability barrier function, facilitating the entry of additional toxins to the adlluminal compartment behind the BTB. The net result thus leads to Sertoli cell and testicular injury. The bottom panel is the black-and-white microgaphs of the green fluorescence microgaphs shown in the top panel, better illustrating the actin microfilaments. Scale bar, 20 μm, which applies to other micrographs.
Microtubule (MT) network
MTs are intracellular filamentous and polarized structures (Figure 1) that are assembled from dimers of two globular proteins α- and β-tubulin that bind in a head-to-fail fashion to form protofilaments with α-tubulin at the minus (−) end and β-tubulin at the positive (+) end [123, 124]. Microtubules with 13 protofilaments surrounding a hollow center are most commonly found in mammalian cells but MTs with 11 or 15 protofilaments also detected in some cell types in mammals and invertebrates [123, 125]. Both ends of a polarized MT can grow via polymerization and can also be depolymerized, but the plus end grows faster and undergoes catastrophe (i.e., switching from growth to shrinking) more frequently [109, 110, 123]. In the testis, the plus- and minus-end of a MT are located to the basal and apical compartment of the seminiferous epithelium, respectively, and they are stabilized by several microtubule-associated proteins (MAPs) such as MARK1-4 (MAP/microtubule affinity regulating kinase 1-4) [126] (Table 4). MTs in eukaryotic cells including Sertoli cells can be as long as 50 μm which can be rapidly reorganized by serving as “rails” to support cell division, endocytic vesicle trafficking, organization of intracellular structure and organelle positioning, ciliary and flagellar motility, spermatid polarity and germ cell transport. Recent studies have shown that MTs likely are working in concert to regulate multiple cellular functions [106, 108], including spermatid transport and endocytic vesicle-mediated protein trafficking. Similar to actin microfilament, MT dynamics are regulated via several classes of proteins (Table 4). First, MT nucleation is mediated by the microtubule-organizing centers (MTOCs), and each MTOC is composed of γ-tubulin and several associated proteins to form a γ-tubulin ring complex (γ-TuRC) that serves as a template for α/β-tubulin dimer to initiate polymerization. Second, MT is regulated by MAPs composed of MAP-1, -2, -3 and -4. The best studied MAP-1 protein is Tau protein which promotes nucleation, preventing MT disassembly, mostly notably involved in the pathogenesis of Alzheimer’s disease [127]. Third, MT depolymerization and cleavage can destabilize MT through the actions of katanin, spastin and fidgetin. For instance, inactivation of katanin p60 (the severing enzyme of katanin) [128] or mutation of katanin p80 (the regulatory subunit of katanin) [129] in mice all led to infertility. Furthermore, some variants of katanin gene (KATNB1) were found to induce oligoasthenotetratozoospermia (OAT) in humans that caused male infertility as manifested by reduced sperm count, abnormal sperm morphology and poor motility [130]. Fourth, MT is also regulated by both plus-end tracking proteins (+TIPs) (e.g., Clip170, EB1) [110] and minus-end tracking proteins (−TIPs) [109] that bind to the tips of growing MTs to regulate MT dynamics. For instance, EB1 (end-binding protein 1, a +TIP) is recently shown to regulate Sertoli cell BTB dynamics by regulating the Sertoli TJ barrier function and the organization of both actin microfilaments and MTs [131], illustrating EB1 is likely one of the cytoskeletal proteins that provide the necessary cross-talks between actin- and MT-based cytoskeletons. Lastly, MT is regulated by motor proteins dynein and kinesin. It is known that dynein is a plus-end MT-dependent motor protein at the Sertoli cell ES [102]. Kinesin is also associated with ES in rat and mouse testes [132]. Both dynein and kinesin are involved in spermatid transport [133]. Exposure of adult rats to 2,5-hexanedione disrupts the distribution of dynein and kinesin, thereby disrupting secretory function of the Sertoli cell in the seminiferous tubule, leading to tubule atrophy [134], causing Sertoli cell injury that leads to germ cell exfoliation. A recent report has shown that deletion of Pih1d3, a PIH1 (protein interacting with Hsp90 1) domain-containing protein that is required for cytoplasmic preassembly of flagellum axonemal dynein in germ cells, leads to male sterility in Pih1d3−/− mice with immotile and fragile spermatozoa [135], illustrating the role of Pih1d3 in proper organization of MT-specific motor protein dynein in microtubules of the spermatid tail. Collectively, these findings illustrate the physiological significance of these MT-specific motor proteins to sperm motility and intracellular protein trafficking in Sertoli cells that support cellular functions in the seminiferous epithelium during spermatogenesis. These observations also illustrate the need of expanding the functionality of this in vitro system in order to monitor the role of MTs in germ cell development, it is obvious that the use a Sertoli-germ cell 3D coculture system as earlier reported [136-138] is needed for future toxicology studies.
Table 4.
Microtubule (MT) binding and regulating proteins*
| Functional type of MT binding proteins |
MT binding or regulating protein |
Localization on MT and functions |
Characteristic domain and binding partner |
Phenotypes of KO and KD |
|---|---|---|---|---|
| MT stabilizing/ polymerizing proteins |
End binding protein family (EB; e.g., EB1, EB2, EB3) |
A +TIP protein, binding to the plus (+) end (i.e., growing end) of MTs [190], induced MT stabilization [191] |
A calponin homology (CH) domain and end binding homology (EBH) domain [192]; interacted with mDia and APC. |
In mouse fibroblasts, EB1 depletion led to a reduced MT growth time [193]; EB1 KD led to an increase in MT dynamics [194] |
| Cytoplasmic linker protein family (CLIP; e.g., CLIP170 CLIP115) |
Binding to the plus end of growing MTs [195], preventing catastrophe or promoting MT rescue events [196, 197] |
Cytoskeleton associated protein/glycine-(CAP-Gly) rich domain mediated the interaction between MTs and EB1 [198] |
Male CLIP-170 KO mice are subfertile, and produced sperm with abnormal heads [199] |
|
| CLIP-associating protein family (CLASP; e.g., CLASP 1, CLASP 2) |
Binding to the MT plus end tracking; stabilizing MTs [200] |
CLASP proteins contained SxIP motifs at central region and TOG domain at the N- terminal region [200, 201] |
The neuromuscular junction in adult Clasp2 KO mice was abnormal due to the mis-localization of CLIP-170 [202]; KD of CLASPs by RNAi led to reduced acetylated tubulin [203] |
|
| Adenomatous polyposis coli family (APC; e.g., APC, APC2 also called APC-L) |
APCs directly associated with MT plus end [204], promoted MT polymerization and stabilization [205] |
Interacted with armadillo repeat domain (ARD) [206], MT interaction domain APCp1, and EB1 interaction domain SxIP [207]. |
APC conditional KO in mice induced accumulation of β-catenin in astroglia; morphological changes in Bergmann glia [208]. |
|
| The tumor overexpressed gene) family (TOG; e.g., ch- TOG) |
TOG proteins localized to MT plus ends by promoting MT elongation [209] |
TOG domain containing protein. The Xenopus TOG, XMAP215, was identified as a MT polymerization promoting protein [210, 211] |
Mice with TOG conditional KO in hippocampal neurons exhibited hyperactivity with impaired short term habituation [212] |
|
| Calmodulin- regulated spectrin- associated protein family (CAMSAP, such as CAMSAP1, CAMSAP2, CAMSAP3) |
A -TIP, CAMSAP bound to the MT minus (−) ends to stabilize MTs against disassembly induced by MT depolymerases [213, 214] |
Presence of C-terminal CKK domain [215] |
PTRN-1 (the C. elegans member of CAMSAP) mutants led to impaired regenerative re-growth of axons; number of dynamic axonal MTs were also induced [216] |
|
| MT severing proteins |
Katanin (katanin p80 or KATNB1, katanin p60) |
Katanin simulated MT plus-end depolymerization [217]. Katanin was shown to be a heterodimer of a p60 severing enzyme and a p80 regulatory subunits [218] |
C-terminal region contained AAA (ATPase associated with diverse cellular activities) ATPase domain; also present is the microtubule interaction and trafficking (MIT) domain [219]; katanin also localized to mitotic spindle poles in mammalian cells that regulated spindle structure and chromosome movement [220] |
Katanin mutation in C. elegans led to failure to form a bipolar spindle [221]; katanin p80 mutation in male mice led to sterility [129]; some genetic variants of KATNB1 also led to oligoasthenoteratozoospermia (typified by low sperm number, abnormal sperm shape and poor motility) in human males [130] |
| Spastin (SPG) | Involved in MT severing at the minus-end [217, 222] |
Contained an AAA ATPase domain, and MIT domain at N-terminal region [223] |
Spastin KO mice were sterile, exhibited progressive axonal degeneration in central nervous system that led to a late and mild motor defect [224]; mutations in SPG4 gene led to autosomal dominant hereditary spastic paraplegia (HSP) in humans [225]. |
|
| Fidgetin (FIGN; consisted of FIGN, FIGNL1, FIGNL2) |
Fidgetin stimulated MT minus-end depolymerization [217] |
Containing AAA ATPase domains [217]. |
Fidgetin KD induced MT-dependent enlargement of mitotic centrosomes and an increase in the number and length of astral MTs [226]. |
|
| MT motor proteins |
Kinesin (there are 45 members of kinesin, composed of 14 classes, and classified into three types based on the relative position of the motor domain) [227] |
A plus-end-directed motor protein [228]; kinesin 8s (Kif18A, Kif18B, Kif19) and kinesin 13s (KIF24, Kif2A, Kif2B and Kif2C/ MCAK) also served as MT depolymerization proteins [229-231] |
Kinesins possessed motor domain and one or more coiled-coil domain [228] |
kif1b−/− KO mice had defects in both sensory- and motor-nerve function, and died at birth due to nervous system defects [232] |
| Dynein | A minus-end-directed motor protein [233], composed of heavy, intermediate, light intermediate, and light chains [234] |
The heavy chain contained the motor domains with six AAA ATPase domains and a MT-binding stalk, dynein interacted with dynactin [234] |
Mutations of several dyneins caused immotile cilia syndrome, human males with immotile cilia syndrome were found to be sterile [235, 236] |
This Table is not intended to be exhaustive, only selected representatives are shown here. Abbreviations used: AAA ATPase domain, ATPase associated with diverse cellular activities ATPase domain; CKK, CAMSAP1, KIAA1078 and KIAA1543 domain which binds to MT; FIGN, fidgetin; FIGNL1, fidgetin-like 1; KD, knockdown; KO, knockout; MT, microtubule; −TIP, microtubule minus-end tracking protein; +TIP, microtubule plus-end tracking protein; SxIP motif, Ser-x-Ile-Pro motif; PTRN-1, PaTRoNin (microtubule-binding protein) homolog 1; RNAi, RNA interference.
Toxicants and Sertoli cell MTs
Studies have shown that MT is a primary target of environmental toxicants in the testis [28, 30, 31]. For instance, the use of toxicants colchicine (an inhibitor of MT polymerization by binding to tubulin which is an alkaloid prepared from dried corns and seeds of Colchicum autumnale) and carbendazim (a fungicide and pesticide) that induce germ cell exfoliation in the testis is mediated by Sertoli MT disruption [139-142]. Carbendazim is also an inhibitor of MT polymerization [143]. Interestingly, the disruptive effects of these toxicants on MT is stage-specific since spermatids that are embedded deep inside into the epithelium near the basal compartment such as in stages I-V tubules are less susceptible to carbendazim toxicity [141]. Furthermore, 2,5-hexanedione (a metabolite of n-hexane which is a component of gasoline, thus low level of environmental exposure in humans is inevitable) induces testicular injury in rodents manifested by disruption of germ cell maturation [28], Sertoli cell vacuolization and germ cell sloughing [31], spermatid head retention [144], inhibition of seminiferous tubule fluid secretion [35], testicular atrophy [145] and an increase in germ cell apoptosis [146] are mediated via a disruption of Sertoli cell MT [28, 31, 147], possibly through an inactivation of MT-specific motor protein function such as dynein and kinesin [134] Collectively, these findings illustrate the physiological significance of MT in seminiferous epithelial homeostasis beyond structural function which include cell apoptosis, fluid secretion, germ cell maturation, and spermatid transport. However, it is of interest to note that there are few studies in the literature that use Sertoli cells cultured in vitro to unfold the mechanism(s) by the toxicants perturb MT function. For instance, it is not known if toxicants mediate their effects via changes in the MT binding/regulatory proteins, +TIPs and/or –TIPs (Table 4), similar to the actin-based cytoskeleton. These studies are important since this information will be helpful to provide clues to therapeutically manage toxicant-induced MT disruption.
Mechanisms by which environment toxicants induce Sertoli cell injury
Altering spatiotemporal expression of ABPs
Earlier studies using Sertoli cells exposed to environmental toxicants (e.g., PBA, cadmium, PFOS) have illustrated considerable changes in the expression of junction proteins, drug transporters, metabolism, and MAPK signaling proteins, including alteration in transcriptome, epigenome, and gene profiling [11, 15, 17-20, 25, 148-150], and some of these important findings are summarized in Table 1. Additionally, studies have also shown that the actin-based cytoskeleton in Sertoli cells is also one of the emerging targets of environmental toxicants (Figure 3), consistent with earlier studies using 2,5-hexanedione and carbendazim as the toxicant (for reviews, see [3, 30, 31] For instance, exposure of rat Sertoli cell to CdCl2 at 3 μM for 3 hr is sufficient to induce defragmentation of actin microfilament in Sertoli cells, and widespread disorganization and disruption of actin filaments are detected by 6 hr [149]. This disruption of actin microfilaments in Sertoli cells induced by toxicants, such as cadmium, BPA or PFOS in either rat Sertoli cells [14, 25, 149] or human Sertoli cells [76] cultured in vitro thus de-stabilize the adhesion proteins complexes at the cell-cell interface, including TJ protein complex occludin-ZO-1, basal ES protein complex N-cadherin-β-catenin, as well connexin43 (Cx43), which utilize actin filaments for attachment (Table 1, Figure 3). Their re-distribution from near the cell surface into the cell cytosol [14, 25, 76], possibly through enhanced endocytosis and/or delayed recycling to cell surface [25] thus perturbs the Sertoli cell TJ-barrier function [14, 25]. Interestingly, treatment of rat or human Sertoli cells with BPA, PFOS or cadmium also affects the spatiotemporal expression of actin bundling proteins palladin or Eps8 (also an actin microfilament barbed end capping protein) near the Sertoli cell surface [14, 76], such that actin microfilaments fail to form bundles at the cortical zone to strength the Sertoli cell TJ-barrier. Cadmium and BPA also induce Arp3 (the branched actin nucleation protein that works with Arp2 to form an Arp2/3 complex, which when activated by N-WASP, this protein complex is capable of inducing branched actin nucleation along an actin microfilament, effectively converting actin microfilaments from a bundled to an unbundled/branched configuration) redistribution in human Sertoli cells, causing Arp3 to retract from cortical zone [76]. Such changes, namely redistribution of Arp3, Eps8 and palladin thus considerably diminish the plasticity of the actin microfilament network at the cortical zone of Sertoli cells, making micofilaments unable to properly re-organize actin filament bundles at the basal ES/BTB to confer TJ-barrier function. More important, using a functional GJ communication assay by FRAP (fluorescence recovery after photobleaching) to track the ability of fluorescence dye transfer through GJ (gap junction) pores between Sertoli cells, both BPA (200 μM) and PFOS (20 μM) at a concentration without detectable cell cytotoxicity based on an XTT assay are found to impair GJ communication [14, 151]. Collectively, these findings suggest that toxicants likely perturb actin-based cytoskeleton by disrupting the spatiotemporal expression of actin bundling proteins (e.g., palladin, Eps8) vs. branched actin-inducing proteins (e.g., Arp3), thereby stripping the ability of actin filament bundles at the ES to convert rapidly between bundled and unbundled/branched configuration to confer cell adhesion and barrier function. These changes are likely linked physiologically to GJ function which provides proper communication between Sertoli and/or germ cells across the seminiferous epithelium so that cellular events across the seminiferous epithelium at a given stage of the epithelial cycle can be tightly coordinated. Herein we provide a likely model by which environmental toxicants induce Sertoli cell injury that perturbs basal ES/BTB function (Figure 3) based on these findings.
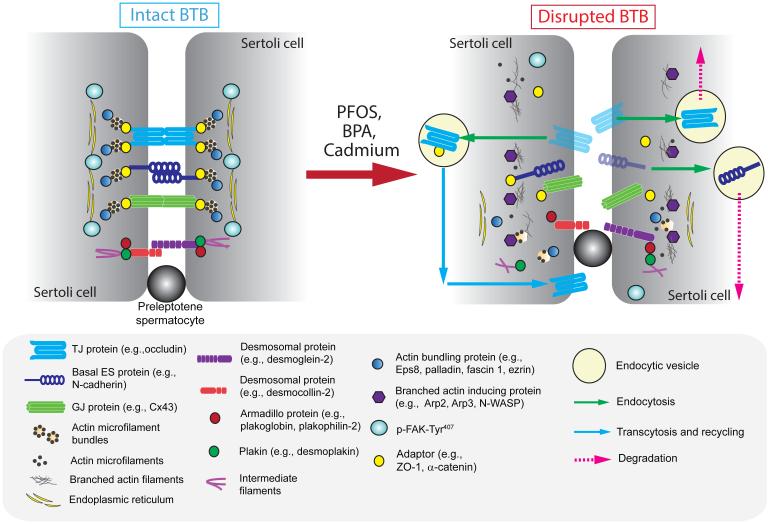
Figure 3. A molecular model by which environmental toxicants induce Sertoli and testicular injury via its initial effects at the BTB.
The left panel illustrates the detailed molecular view of an intact BTB, such as at stage VII of the epithelial cycle in the rat testis, which is conferred by the adhesion protein complexes of the actin-based TJ, basal ES and GJ, as well as the intermediate filament based desmosome proteins between adjacent Sertoli cells. As shown in Figure 1, one of the most typical features of the BTB is the prominent presence of actin microfilaments that aligned perpendicular to the Sertoli cell plasma membrane and are sandwiched in-between the cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane. This array of actin microfilament bundles supported by the enhanced expression of actin bundling proteins (e.g., Eps8 also barbed end capping protein, palladin, plastin 3, fascin 1) and p-FAK-Tyr407, and a reduced expression of branched actin polymerization protein Arp3, also confer the strong adhesive strength of the BTB, making it one of the tighest blood-tessue barriers in the mammalian body. However, exposure of the Sertoli cells isolated from either rodents or humans to the environmental toxicants, such as PFOS, BPA and cadmium as discussed in text, with an established TJ-permeability barrier that mimics the BTB in vivo, these toxicants rapidly alter the spatiotemporal expression of two major groups of actin binding proteins (APBs), such as (i) actin barbed end capping and bundling protein Eps8 or other actin bundling/cross-linking proteins (e.g., palladin, plastin 3, fascin 1, ezrin) that confer actin microfilaments their bundled configuration, and (ii) branched acin polymerization protein Arp3. In short, a reduced expression of actin bundling proteins and p-FAK-Tyr407 are noted, concomitant with an up-regulation of Arp3 at nor near the Sertoli cell-cell interface. The net result in turn destabilizes adhesion protein complexes are the site, either undergo endosome-mediated protein degradation or transcytosis and recycling. This thus facilitates the entry of more environmental toxicants to the adluminal compartment, perturbing either meiosis I/II or post-meiotic spermatid development, spermatid transport and spermatid polarity.
Altering expression of p-FAK-Tyr407 and/or p-FAK-Tyr397
Recent studies have shown that the two phosphorylated forms of FAK, p-FAK-Tyr407 and p-FAK-Tyr397 are crucial regulators of ES function in the testis via their effects on the actin microfilaments [152, 153]. Their role on actin microfilament organization at the ES mediated through ABPs, such as Arp3, Eps8, and palladin, is supported by two recent reports. First, overexpression of a p-FAK-Tyr397 phosphomimetic mutant FAK Y397E in the testis in vivo has been shown to delay F-actin degeneration at the apical ES that leads to a retention of elongated spermatids in the seminiferous epithelium in late stage VIII tubules [153]. This phenotype is caused by an impairment on the spatiotemporal expression of Arp3, Eps8 and palladin, since these proteins should have been considerably down-regulated in stage VIII tubules that allow degeneration of apical ES to facilitate the release of sperm at spermiation, yet they remain considerably expressed at the apical ES to support spermatid adhesion [153]. This thus retains apical ES-specific adhesion proteins nectin-2 and nectin-3 [154] at the apical ES since F-actin remains for their attachment [153]. Second, overexpression of a constitutively active p-FAK-Tyr407 phosphomimetic mutant (FAK Y407E) in Sertoli cells cultured in vitro has been shown to promote the TJ-barrier, making it tighter [152]. This promoting effect of FAK Y407E mutant on the basal ES/BTB in Sertoli cells is mediated, at least in part, via an increase in actin polymerization through the action of Arp3 at the cortical zone [152], by conferring plasticity to actin microfilament bundles at the basal ES. Consistent with these findings, overexpression of FAK Y407E in rat Sertoli cell epithelium in vitro with an established TJ-permeability barrier is capable of tightening the TJ-barrier, making it less responsive to the disruptive effects of PFOS on the barrier function [14]. This protective effect is mediated by re-organization of actin microfilaments at the cortical zone so that more actin microfilaments are found near the Sertoli cell-cell interface [14]. This finding is also in agreement with an earlier report that a knockdown of FAK, by RNAi using FAK-specific siRNA duplexes vs. non-targeting negative control siRNA duplexes, caused Sertoli cells cultured in vitro to be more sensitive to cadmium treatment, making the Sertoli cell TJ-barrier more susceptible to the disruptive effects of cadmium [43]. Collectively, these data suggest that p-FAK-Tyr397 and p-FAK-Tyr407 are crucial regulators of actin microfilaments at the ES, and they likely exert their effects on the intrinsic activity of Arp3, Eps8 and/or palladin (Figure 3), plausibly via direct phosphorylation to activate these ABPs. These findings also implicate the possibility that these two FAK isoforms are useful therapeutic candidates to manage environmental toxicant-mediated Sertoli cell injury, such as via their overexpression using gene therapy approach as reported in men [155] and rodents [156] following industrial or accidental exposure to acute dose of some selected toxicants.
Concluding remarks, questions, and future perspectives
It is increasingly clear that environmental toxicants induce testicular injury via their effects on the Sertoli cell cytoskeletons. For instance, toxicants perturb the spatiotemporal expression of actin- and/or MT-binding and regulatory proteins in Sertoli cells, thereby altering the organization of actin microfilaments and/or MT filaments. These changes thus reduce the plasticity of these cytoskeletal elements, making them incapable of responding to changes during the epithelial cycle of spermatogenesis to support germ cell transport and/or intracellular protein/organelle trafficking, among other events. Alternatively, these changes also cause truncation of cytoskeletal filaments, leading to the eventual exfoliation of germ cells. We provide herein a hypothetical mechanism (Figure 3) regarding the toxicant-mediated BTB dysfunction. This model also illustrates that by changing the spatiotemporal expression of actin regulatory proteins such as Arp2/3 complex and Eps8 to re-organize actin microfilaments at the BTB can support preleptotene spermatocyte transport at the BTB. Thus, the use of toxicant-induced Sertoli cell injury in vitro is a useful model to probe the molecular mechanisms that regulate spermatogenesis.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (NICHD, R01 HD056034 to C.Y.C., and U54 HD029990, Project 5 to C.Y.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ roles
C.Y.C. prepared the first draft of the manuscript, N.L. and C.Y.C. performed the literature search, research on the topic and critically evaluated published findings in the literature; C.Y.C., N.L., and D.D.M. critically discussed and evaluated the latest research and findings in the field pertinent to the topic; N.L. and C.Y.C. prepared the figures; N.L., D.D.M., W.M.L. C.K.C.W. and C.Y.C. critically evaluated the information and published data discussed in this manuscript; N.L. and C.Y.C. performed the final editing of the manuscript; all authors approved the final version of the manuscript.
Conflicts of interest
None declared.
References
- [1].Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28:462–70. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Toft G, Jonsson BA, Lindh CH, Giwercman A, Spano M, Heederik D, et al. Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Human Reprod. 2012;27:2532–40. doi: 10.1093/humrep/des185. [DOI] [PubMed] [Google Scholar]
- [3].Boekelheide K. Sertoli cell toxicants. In: Russell L, Griswold M, editors. The Sertoli Cell. Cache River Press; Clearwater: 1993. pp. 551–75. [Google Scholar]
- [4].Boekelheide K. Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr. 2005;34:6–8. doi: 10.1093/jncimonographs/lgi006. [DOI] [PubMed] [Google Scholar]
- [5].Okanlawon A, Dym M. Effect of chloroquine on the formation of tight junctions in cultured immature rat Sertoli cells. J Androl. 1996;17:249–55. [PubMed] [Google Scholar]
- [6].Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures - a new model for toxicological investigations of the "blood-testis" barrier in vitro. Toxicol Appl Pharmacol. 1992;112:51–7. doi: 10.1016/0041-008x(92)90278-z. [DOI] [PubMed] [Google Scholar]
- [7].Chen J, Fok KL, Chen H, Zhang XH, Xu WM, Chan HC. Cryptorchidism-induced CFTR down-regulation results in disruption of testicular tight junctions through up-regulation of NF-κB/COX-2/PGE2. Hum Reprod. 2012;27:2585–97. doi: 10.1093/humrep/des254. [DOI] [PubMed] [Google Scholar]
- [8].Heindel J, Chapin R. Inhibition of FSH-stimulated cAMP accumulation by mono(2-ethylhexyl) phthalate in primary rat Sertoli cell cultures. Toxicol Appl Pharmacol. 1989;97:377–85. doi: 10.1016/0041-008x(89)90342-6. [DOI] [PubMed] [Google Scholar]
- [9].Heindel JJ, Powell CJ. Phthalate ester effects on rat Sertoli cell function in vitro: effects of phthalate side chain and age of animal. Toxicol Appl Pharmacol. 1992;115:116–23. doi: 10.1016/0041-008x(92)90374-2. [DOI] [PubMed] [Google Scholar]
- [10].Nicholls PK, Harrison CA, Gilchrist RB, Farnworth PG, Stanton PG. Growth differentiation factor 9 is a germ cell regulator of Sertoli cell function. Endocrinology. 2009;150:2481–90. doi: 10.1210/en.2008-1048. [DOI] [PubMed] [Google Scholar]
- [11].Chung NPY, Cheng CY. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–88. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- [12].Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- [13].Du M, Young J, De Asis M, Cipollone J, Roskelley C, Takai Y, et al. A novel subcellular machine contributes to basal junction remodeling in the seminiferous epithelium. Biol Reprod. 2013;88:60. doi: 10.1095/biolreprod.112.104851. [DOI] [PubMed] [Google Scholar]
- [14].Wan HT, Mruk DD, Wong CKC, Cheng CY. Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr407 - an in vitro study. Endocrinology. 2014;155:249–62. doi: 10.1210/en.2013-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Qiu L, Zhang X, Zhang X, Zhang Y, Gu J, Chen M, et al. Sertoli cell is a potential target for perfluorooctane sulfonate-induced reproductive dysfunction in male mice. Toxicol Sci. 2013;135:229–40. doi: 10.1093/toxsci/kft129. [DOI] [PubMed] [Google Scholar]
- [16].Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–5. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Syed V, Gu W, Hecht NB. Sertoli cells in culture and mRNA differntial display provide a sensitive early warning assay system to detect changes induced by xenobiotics. J Androl. 1997;18:264–74. [PubMed] [Google Scholar]
- [18].Gualtieri AF, Iwachow MA, Venara M, Rey RA, Schteingart HF. Bisphenol A effect on glutathione synthesis and recycling in testicular Sertoli cells. J Endocrinol Invest. 2011;34:e102–e9. doi: 10.1007/BF03347468. [DOI] [PubMed] [Google Scholar]
- [19].Fiorini C, Tilloy-Ellul A, Chevalier S, Charuel C, Pointis G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod Toxicol. 2004;18:413–21. doi: 10.1016/j.reprotox.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [20].Bekheet SH, Stahlmann R. Disruption of gap junctional intercellular communication by antibiotic gentamicin is assoicated with aberrant localization of occludin, N-cadherin, connexin 43, and vimentin in SerW3 Sertoli cells in vitro. Environ Toxicol Pharmacol. 2009;28:155–60. doi: 10.1016/j.etap.2009.03.002. [DOI] [PubMed] [Google Scholar]
- [21].Yu X, Hong S, Moreira EG, Faustman EM. Improving in vitro Sertoli cell/gonocyte co-culture model for assessing male reproductive toxicity: Lessons learned from comparisons of cytotoxicity versus genomic responses to phthalates. Toxicol Appl Pharmacol. 2009;239:325–36. doi: 10.1016/j.taap.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–42. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- [23].Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–98. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- [24].Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Communs. 2012;3:1185. doi: 10.1038/ncomms2171. (doi:10.038/ncomms2171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–14. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thuillier R, Manku G, Wang Y, Culty M. Changes in MAPK pathway in neonatal and adult testis following fetal estrogen exposure and effects on rat testicular cells. Microsc Res Tech. 2009;72:773–86. doi: 10.1002/jemt.20756. [DOI] [PubMed] [Google Scholar]
- [27].Li MWM, Mruk DD, Cheng CY. Mitogen-activated protein kinases in male reproductive function. Trends Mol Med. 2009;15:159–68. doi: 10.1016/j.molmed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boekelheide K, Fleming SL, Allio T, Embree-Ku ME, Hall SJ, Johnson KJ, et al. 2,5-Hexanedione-induced testicular injury. Annu Rev Pharmacol Toxciol. 2003;43:125–47. doi: 10.1146/annurev.pharmtox.43.100901.135930. [DOI] [PubMed] [Google Scholar]
- [29].Boekelheide K, Johnson KJ, Richburg JH. Sertoli cell toxicants. In: Skinner MK, Griswold MD, editors. Sertoli Cell Biology. Elsevier Science; New York: 2005. pp. 345–382. [Google Scholar]
- [30].Boekelheide K, Neely MD, Sioussat TM. The Sertoli cell cytoskeleton: a target for toxicant-induced germ cell loss. Toxicol Appl Pharmacol. 1989;101:373–89. doi: 10.1016/0041-008x(89)90188-9. [DOI] [PubMed] [Google Scholar]
- [31].Johnson KJ. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis. 2014;4:e979106. doi: 10.4161/21565562.2014.979106. (DOI:10.4161/21565562.2014.979106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].O'Donnell L. Mechanisms of spermatogenesis and spermiation and how they are disturbed. Spermatogenesis. 2014;4:e979623. doi: 10.4161/21565562.2014.979623. (DOI:10.4161/21565562.2014.979623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Blanchard KT, Allard EK, Boekelheide K. Fate of germ cells in 2,5-hexanedione-induced testicular injury. I. Apoptosis is the mechanism of germ cell death. Toxicol Appl Pharmacol. 1996;137:141–8. doi: 10.1006/taap.1996.0066. [DOI] [PubMed] [Google Scholar]
- [34].Allard EK, Boekelheide K. Fate of germ cells in 2,5-hexanedione-induced testicular injury. Toxicol Appl Pharmacol. 1996;137:149–56. doi: 10.1006/taap.1996.0067. [DOI] [PubMed] [Google Scholar]
- [35].Johnson KJ, Hall ES, Boekelheide K. 2,5-Hexanedione exposure alters the rat Sertoli cell cytoskeleton. I. Microtubules and seminifersous tubule fluid secretion. Toxicol Appl Pharmacol. 1991;111:432–42. doi: 10.1016/0041-008x(91)90248-d. [DOI] [PubMed] [Google Scholar]
- [36].Hall ES, Hall SJ, Boekelheide K. Sertoli cells isolated from adult 2,5-hexanedione-exposed rats exhibit atypical morphology and actin distribution. Toxicol Appl Pharmacol. 1992;117:9–18. doi: 10.1016/0041-008x(92)90211-a. [DOI] [PubMed] [Google Scholar]
- [37].Hall ES, Eveleth J, Boekelheide K. 2,5-Hexanedione exposure alters the rat Sertoli cell cytoskeleton. II. Intermediate filaments and actin. Toxicol Appl Pharmacol. 1991;111:443–53. doi: 10.1016/0041-008x(91)90249-e. [DOI] [PubMed] [Google Scholar]
- [38].WHO . Air Quality Guidelines. World Health Organization; Regional Office for Europe, Copenhagen, Denmark: 2000. Cadmium. [Google Scholar]
- [39].Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds - exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;212:239–70. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- [41].Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005-2006 National Health and Nutrition Examinaiton Survey. J Expo Sci Environ Epidemiol. 2011;21:272–9. doi: 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilenblum W, Schweinfurth H, et al. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol. 2011;41:263–91. doi: 10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Siu ER, Wong EWP, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA. 2009;106:9298–303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].O'Donnell L, O'Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol. 2014;30:45–54. doi: 10.1016/j.semcdb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- [45].Cheng CY. Toxicants target cell junctions in the testis - insights from the indazole-carboxylic acid model. Spermatogenesis. 2014;4:e981485. doi: 10.4161/21565562.2014.981485. (DOI:10.4161/21565562.2014.9814895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vidal JD, Whitney KM. Morphologic manifestations of testicular and epididymal toxicity. Spermatogenesis. 2014;4:e979099. doi: 10.4161/21565562.2014.979099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Parizek J, Zahor Z. Effect of cadmium salts on testicular tissue. Nature. 1956;177:1036–7. doi: 10.1038/1771036b0. [DOI] [PubMed] [Google Scholar]
- [48].Parizek J. The destructive effect of cadmium ion on testicular tissue and its prevention by zinc. J Endocrinol. 1957;15:56–63. doi: 10.1677/joe.0.0150056. [DOI] [PubMed] [Google Scholar]
- [49].Gunn SA, Gould TC, Anderson WAD. The selective injurious response of testicular and epididymal blood vessels to cadmium and its prevention by zinc. Am J Pathol. 1963;42:685–702. [PMC free article] [PubMed] [Google Scholar]
- [50].Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the "blood-testis barrier" after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–6. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- [51].Wong CH, Mruk DD, Siu MKY, Cheng CY. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- [52].Elkin ND, Piner JA, Sharpe RM. Toxicant-induced leakage of germ cell-specific proteins from seminiferous tubules in the rat: relationship to blood-testis barrier integrity and prospects for biomonitoring. Toxicol Sci. 2010;117:439–48. doi: 10.1093/toxsci/kfq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol. 2011;763:237–52. doi: 10.1007/978-1-61779-191-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Perryman K, Stanton P, Loveland K, McLachlan R, Robertson D. Hormonal dependency of neural cadherin in the binding of round spermatids to Sertoli cells in vitro. Endocrinology. 1996;137:3877–83. doi: 10.1210/endo.137.9.8756560. [DOI] [PubMed] [Google Scholar]
- [55].Lampa J, Hoogerbrugge J, Baarends W, Stanton P, Perryman K, Grootegoed J, et al. Follicle-stimulating hormone and testosterone stimulation of immature and mature Sertoli cells in vitro: inhibin and N-cadherin levels and round spermatid binding. J Androl. 1999;20:399–406. [PubMed] [Google Scholar]
- [56].Okuma Y, O'Connor AE, Muir JA, Stanton PG, De Kretser DM, Hedger MP. Regulation of activin A and inhibin B secretion by inflamatory mediators in adult rat Sertoli cell cultures. J Endocr. 2005;187:125–34. doi: 10.1677/joe.1.06266. [DOI] [PubMed] [Google Scholar]
- [57].Nicholls PK, Stanton PG, Chen JL, Olcorn JS, Haverfield JT, Qian H, et al. Activin signaling regulates Sertoli cell differentiation and function. Endocrinology. 2012;153:6065–77. doi: 10.1210/en.2012-1821. [DOI] [PubMed] [Google Scholar]
- [58].Reis MM, Moreira AC, Sousa M, Mathur PP, Oliveira PF, Alves MG. Sertoli cell as a model in male reproductive toxicology: Advantages and disadvantages. J Appl Toxicol. 2015;35:870–83. doi: 10.1002/jat.3122. [DOI] [PubMed] [Google Scholar]
- [59]. www.researchdiets.com/resource-center-page/typical-food-intake.
- [60].Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl. 2008;29:469–87. doi: 10.2164/jandrol.107.004655. [DOI] [PubMed] [Google Scholar]
- [61].Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- [62].Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology. 2014;29:286–98. doi: 10.1152/physiol.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction. 2006;132:673–80. doi: 10.1530/rep.1.01081. [DOI] [PubMed] [Google Scholar]
- [64].Schlatt S, Ehmcke J. Regulation of spermatogenesis: an evolutionary biologist's perspective. Semin Cell Dev Biol. 2014;29:2–16. doi: 10.1016/j.semcdb.2014.03.007. [DOI] [PubMed] [Google Scholar]
- [65].Robaire B. Advancing towards a male contraceptive: a novel approach from an unexpected direction. Trends Pharmacol Sci. 2003;24:326–8. doi: 10.1016/S0165-6147(03)00141-X. [DOI] [PubMed] [Google Scholar]
- [66].Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Griem P, Hassauer M, Kalberlah F, Oltmanns J, Scheibner J, Schneider K, et al. Final Report of Project F 1656. Federal Institute for Occupational Safety and Health; Dortmund, Germany: 2002. Quantitative differences in xenobiotic metabolism between experimental animals and humans; pp. 1–13. [Google Scholar]
- [68].Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–94. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- [69].Luttrell WE, Castle MC. Species differences in the hydrolysis of meperidine and its inhibition by organophosphate compounds. Fundam Appl Toxicol. 1988;11:323–32. doi: 10.1016/0272-0590(88)90157-1. [DOI] [PubMed] [Google Scholar]
- [70].Kudo NK. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci. 2003;28:49–57. doi: 10.2131/jts.28.49. Y. [DOI] [PubMed] [Google Scholar]
- [71].Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Archives of environmental contamination and toxicology. 2009;56:338–49. doi: 10.1007/s00244-008-9194-6. [DOI] [PubMed] [Google Scholar]
- [72].Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: A quantitative autoradiographic study. Anat Rec. 1982;203:485–92. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- [73].Li JCH, Lee WM, Mruk DD, Cheng CY. Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro. J Androl. 2001;22:266–77. [PubMed] [Google Scholar]
- [74].Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, et al. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod. 2009;80:1084–91. doi: 10.1095/biolreprod.108.071662. [DOI] [PubMed] [Google Scholar]
- [75].Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh ALT, et al. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant. 2011;20:619–35. doi: 10.3727/096368910X536563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Xiao X, Mruk DD, Tang EI, Wong CKC, Lee WM, John CM, et al. Environmental toxicants perturb human Serotli cell adhesive function via changes in F-actin organization medicated by actin regulatory proteins. Hum Reprod. 2014;29:1279–91. doi: 10.1093/humrep/deu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pognan F, Masson MT, Lagelle F, Charuel C. Establishment of a rat Sertoli cell line that displays the morphological and some of the functional characteristics of the native cell. Cell Biol Toxicol. 1997;13:453–63. doi: 10.1023/a:1007475928452. [DOI] [PubMed] [Google Scholar]
- [78].McGuinness MP, Linder CC, Morales Cr, Heckert LL, Pikus J, Griswold MD. Relationship of a mouse Sertoli cell line (MSC-1) to normal Sertoli cells. Biol Reprod. 1994;51:116–24. doi: 10.1095/biolreprod51.1.116. [DOI] [PubMed] [Google Scholar]
- [79].Peschon JJ, Behringer RR, Cate RL, Harwood KA, Idzerda RL, Brinster RL, et al. Directed expression of an oncogene to Sertoli cells in transgenic mice using Mullerian inhibiting substance regulatory sequences. Mol Endocrinol. 1992;6:1403–11. doi: 10.1210/mend.6.9.1331774. [DOI] [PubMed] [Google Scholar]
- [80].Kaur G, Dufour JM. Cell lines. Valuable tools or useless artifacts. Spermatogenesis. 2012;2:1–5. doi: 10.4161/spmg.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mruk DD, Zhu LJ, Silvestrini B, Lee WM, Cheng CY. Interactions of proteases and protease inhibitors in Sertoli-germ cell cocultures preceding the formation of specialized Sertoli-germ cell junctions in vitro. J Androl. 1997;18:612–22. [PubMed] [Google Scholar]
- [82].Clermont Y, Morales C, Hermo L. Endocytic activities of Sertoli cells in the rat. Ann NY Acad Sci. 1987;513:1–15. doi: 10.1111/j.1749-6632.1987.tb24994.x. [DOI] [PubMed] [Google Scholar]
- [83].Cheng CY, Mruk DD. Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium. In: Griswold MD, editor. Sertoli Cell Biology. 2nd. Elsevier; Amsterdam: 2015. pp. 333–383. DOI: http://dx.doi.org/10.1016/B978-0-12-417047-6.00012.0. [Google Scholar]
- [84].Weber JE, Russell LD, Wong V, Peterson RN. Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat. 1983;167:163–79. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- [85].Boussouar F, Benahmed M. Lactate and energy metabolism in male germ cells. Trends Endocrinol Metab. 2004;15:345–50. doi: 10.1016/j.tem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- [86].Rato L, Alves MG, Socorro S, Durate AI, Cavaco JE, Oliveira PF. Metabolic regulation is important for spermatogenesis. Nat Rev Urol. 2012;9:330–8. doi: 10.1038/nrurol.2012.77. [DOI] [PubMed] [Google Scholar]
- [87].Li MWM, Mruk DD, Cheng CY. Gap junctions and blood-tissue barriers. Adv Exp Med Biol. 2012;763:260–80. doi: 10.1007/978-1-4614-4711-5_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Gungor-Ordueri NE, Tang EI, Celik-Ozenci C, Cheng CY. Ezrin is an actin binding protein that regulates Sertoli cell and spermatid adhesion during spermatogenesis. Endocrinology. 2014;155:3981–95. doi: 10.1210/en.2014-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gerdes HH, Carvalho RN. Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20:470–5. doi: 10.1016/j.ceb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- [90].Gerdes HH, Rustom A, Wang X. Tunneling nanotubes, an emerging intercellular communication route in development. Mech Dev. 2013;130:381–7. doi: 10.1016/j.mod.2012.11.006. [DOI] [PubMed] [Google Scholar]
- [91].O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].O’Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis. 2014;4:e979623. doi: 10.4161/21565562.2014.979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94:235–63. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- [94].Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315–33. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Oda T, Iwasa M, Aihara T, Maeda Y, Narita A. The nature of the globular-to fibrous-actin transition. Nature. 2009;457:441–5. doi: 10.1038/nature07685. [DOI] [PubMed] [Google Scholar]
- [96].De La Cruz EM, Gardel ML. Actin mechanics and fragmentation. J Biol Chem. 2015;290:17137–44. doi: 10.1074/jbc.R115.636472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kierszenbaum AL, Rivkin E, Tres LL. The actin-based motor myosin Va is a component of the acroplaxome, an acrosome-nuclear envelope junctional plate, and of manchette-associated vesicles. Cytogenet Genome Res. 2003;103:337–44. doi: 10.1159/000076822. [DOI] [PubMed] [Google Scholar]
- [98].Hayasaka S, Terada Y, Suzuki K, Murakawa H, Tachibana I, Sankai T, et al. Intramanchette transport during primate spermiogenesis: expression of dynein, myosin Va, motor recruiter myosin Va,VIIa-Rab27a/b interacting protein, and Rab27b in the manchette during human and monkey spermiogenesis. Asian J Androl. 2008;10:561–8. doi: 10.1111/j.1745-7262.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- [99].Noguchi T, Lenartowska M, Miller KG. Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Mol Biol Cell. 2006;17:2559–71. doi: 10.1091/mbc.E06-01-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hasson T, Walsh J, Cable J, Mooseker M, Brown S, Steel K. Effects of shaker-1 mutations on myosin-VIIa protein and mRNA expression. Cell Motil Cytoskeleton. 1997;37:127–38. doi: 10.1002/(SICI)1097-0169(1997)37:2<127::AID-CM5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- [101].Yang F, Wei Q, Adelstein RS, Wang PJ. Non-muscle myosin IIB is essential for cytokinesis during male meiotic cell division. Dev Biol. 2012;369:356–61. doi: 10.1016/j.ydbio.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Guttman JA, Kimel GH, Vogl AW. Dynein and plus-end microtubule-dependent motors are associated with specialized Sertoli cell junction plaques (ectoplasmic specializations) J Cell Sci. 2000;113:2167–76. doi: 10.1242/jcs.113.12.2167. [DOI] [PubMed] [Google Scholar]
- [103].Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development. Human Reprod Update. 2004;10:349–69. doi: 10.1093/humupd/dmh026. [DOI] [PubMed] [Google Scholar]
- [104].Echarri A, Del Pozo MA. Caveolae - mechanosensitive membrane invaginations linked to actin filaments. J Cell Sci. 2015;128:2747–58. doi: 10.1242/jcs.153940. [DOI] [PubMed] [Google Scholar]
- [105].Kierszenbaum AL, Rivkin E, Tres LL. Cytoskeletal track selection during cargo transport in spermatids is relevant to male fertility. Spermatogenesis. 2011;1:221–30. doi: 10.4161/spmg.1.3.18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Coles CH, Bradke F. Coordinating Neuronal Actin-Microtubule Dynamics. Curr Biol. 2015;25:R677–91. doi: 10.1016/j.cub.2015.06.020. [DOI] [PubMed] [Google Scholar]
- [107].Poulter NS, Thomas SG. Cytoskeletal regulation of platelet formation: Coordination of F-actin and microtubules. Int J Biochem Cell Biol. 2015;66:69–74. doi: 10.1016/j.biocel.2015.07.008. [DOI] [PubMed] [Google Scholar]
- [108].Schappi JM, Krbanjevic A, Rasenick MM. Tubulin, actin and heterotrimeric G proteins: coordination of signaling and structure. Biochim Biophys Acta. 2014;1838:674–81. doi: 10.1016/j.bbamem.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Akhmanova A, Hoogenraad CC. Microtubule minus-end-targeting proteins. Curr Biol. 2015;25:R162–71. doi: 10.1016/j.cub.2014.12.027. [DOI] [PubMed] [Google Scholar]
- [110].Akhmanova A, Steinmetz MO. Microtubule +TIPs at a glance. J Cell Sci. 2010;123:3415–9. doi: 10.1242/jcs.062414. [DOI] [PubMed] [Google Scholar]
- [111].Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol. 2013;217:R13–R23. doi: 10.1530/JOE-12-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Qian X, Mruk DD, Cheng YH, Tang EI, Han D, Lee WM, et al. Actin binding proteins, spermatid transport and spermiation. Semin Cell Dev Biol. 2014;30:75–85. doi: 10.1016/j.semcdb.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–92. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- [115].Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- [116].Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–67. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology. 2013;154:1907–20. doi: 10.1210/en.2012-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Li N, Mruk DD, Wong CK, Lee WM, Han D, Cheng CY. Actin-bundling protein plastin 3 is a regulator of ectoplasmic specialization dynamics during spermatogenesis in the rat testis. FASEB J. 2015;29:3788–805. doi: 10.1096/fj.14-267997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gungor-Ordueri NE, Celik-Ozenci C, Cheng CY. Fascin 1 is an actin filament-bundling protein that regulates ectoplasmic specialization dynamics in the rat testis. Am J Physiol Endocrinol Metab. 2014;307:E738–E53. doi: 10.1152/ajpendo.00113.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–62. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Desai A, Mitochison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- [124].Tang EI, Mruk DD, Lee WM, Cheng CY. Cell-cell interactions, cell polarity, and the blood-testis barrier. In: Ebnet K, editor. Cell Polarity 1. Springer International Publishing; Geneva: 2015. pp. 303–326. DOI: 101007/978-3-319-14463-4_13. [Google Scholar]
- [125].Cueva JG, Hsin J, Huang KC, Goodman MB. Posttranslational acetylation of α-tubulin constrains protofilament number in native microtubules. Curr Biol. 2012;22:1066–74. doi: 10.1016/j.cub.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Tang EI, Xiao X, Mruk DD, Qian XJ, Mok KW, Jenardhanan P, et al. Microtubule affinity-regulating kinase 4 (MARK4) is a component of the ectoplasmic specialization in the rat testis. Spermatogenesis. 2012;2:117–26. doi: 10.4161/spmg.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Medina M, Avila JL. The role of extracellular Tau in the spreading of neurofibrillary pathology. Front Cell Neurosci. 2014;8:113. doi: 10.3389/fncel.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Smith LB, Milne L, Nelson N, Eddie S, Brown P, Atanassova N, et al. KATNAL1 regulation of Sertoli cell microtubule dynamics is essential for spermiogenesis and male fertility. PLoS genetics. 2012;8:e1002697. doi: 10.1371/journal.pgen.1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].O'Donnell L, Rhodes D, Smith SJ, Merriner DJ, Clark BJ, Borg C, et al. An essential role for katanin p80 and microtubule severing in male gamete production. PLoS genetics. 2012;8:e1002698. doi: 10.1371/journal.pgen.1002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].O'Donnell L, McLachlan RI, Merriner DJ, O'Bryan MK, Jamsai D. KATNB1 in the human testis and its genetic variants in fertile and oligoasthenoteratozoospermic infertile men. Andrology. 2014;2:884–91. doi: 10.1111/andr.276. [DOI] [PubMed] [Google Scholar]
- [131].Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the Sertoli cell blood-testis barrier in male rats - an in vitro study. Endocrinology. 2015;156:680–93. doi: 10.1210/en.2014-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Vaid KS, Guttman JA, Singaraja RR, Vogl AW. A kinesin is present at unique Sertoli/spermatid adherens junctions in rat and mouse testes. Biol Reprod. 2007;77:1037–48. doi: 10.1095/biolreprod.107.063735. [DOI] [PubMed] [Google Scholar]
- [133].Miller M, Mulholland D, Vogl A. Rat testis motor proteins associated with spermatid translocation (dynein) and spermatid flagella (kinesin-II) Biol Reprod. 1999;60:1047–56. doi: 10.1095/biolreprod60.4.1047. [DOI] [PubMed] [Google Scholar]
- [134].Hall ES, Hall SJ, Boekelheide K. 2,5-Hexanedione exposure alters microtubule motor distribution in adult rat testis. Fundam Appl Toxicol. 1995;24:173–82. doi: 10.1006/faat.1995.1021. [DOI] [PubMed] [Google Scholar]
- [135].Dong F, Shinohara K, Botilde Y, Nabeshima R, Asai Y, Fukumoto A, et al. Pih1d3 is required for cytoplasmic preassembly of axonemal dynein in mouse sperm. J Cell Biol. 2014;204:203–13. doi: 10.1083/jcb.201304076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Stukenborg JB, Wistuba J, Luetjens CM, Elhija MA, Huleihel M, Lunenfeld E, et al. Coculture of spermatogonia with somatic cells in a novel three-dimensional soft-agar-culture-system. J Androl. 2008;29:312–29. doi: 10.2164/jandrol.107.002857. [DOI] [PubMed] [Google Scholar]
- [137].Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–7. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- [138].Sato T, Katagiri K, Yoknishi T, Kuboto Y, Inoue K, Ogonuki N, et al. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun. 2011;2:472. doi: 10.1038/ncomms1478. (DOI:10.1038/ncomms1478) [DOI] [PubMed] [Google Scholar]
- [139].Allard EK, Johnson KJ, Boekelheide K. Colchicine disrupts the cytoskeleton of rat testis seminiferous epithelium in a stage-dependent manner. Biol Reprod. 1993;48:143–53. doi: 10.1095/biolreprod48.1.143. [DOI] [PubMed] [Google Scholar]
- [140].Nakai M, Hess RA. Morphological changes in the rat Sertoli cell induced by the microtubule poison carbendazim. Tissue Cell. 1994;26:917–27. doi: 10.1016/0040-8166(94)90041-8. [DOI] [PubMed] [Google Scholar]
- [141].Nakai M, Miller MG, Carnes K, Hess RA. Stage-specific effects of the fungicide carbendazim on Sertoli cell microtubules in rat testis. Tissue Cell. 2002;34:73–80. doi: 10.1016/s0040-8166(02)00006-x. [DOI] [PubMed] [Google Scholar]
- [142].Lim JP, Miller MG. The role of the benomyl metabolite carbendazim in benomyl-induced testicular toxicity. Toxicol Appl Pharmacol. 1997;142:401–10. doi: 10.1006/taap.1996.8042. [DOI] [PubMed] [Google Scholar]
- [143].Winder BS, Strandgaard CS, Miller MG. The role of GTP binding and microtubule-associated proteins in the inhibition of microtubule assembly by carbendazim. Toxicol Sci. 2001;59:138–46. doi: 10.1093/toxsci/59.1.138. [DOI] [PubMed] [Google Scholar]
- [144].Bryant BH, Yamasaki H, Sandrof MA, Boekelheide K. Spermatid head retention as a marker of 2,5-hexanedione-induced testicular toxicity in the rat. Toxciol Pathol. 2008;36:552–9. doi: 10.1177/0192623308317426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Boekelheide K, Hall SJ. 2,5-Hexanedione exposure in the rat results in long-term testicular atrophy despite the presence of residual spermatogonia. J Androl. 1991;12:18–26. [PubMed] [Google Scholar]
- [146].Yamasaki H, Sandrof MA, Boekelheider K. Suppression of radiation-induced testicular germ cell apoptosis by 2,5-hexanedione pretreatment. I. Histopathological analysis reveals stage dependence of attenuated apoptosis. Toxicol Sci. 2010;117:449–56. doi: 10.1093/toxsci/kfq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Markelewicz RJJ, Hall SJ, Boekelheide K. 2,5-hexanedione and carbendazim coexposure synergistically disrupts rats spermatogenesis despite opposing molecular effects on microtubules. Toxciol Sci. 2004;80:92–100. doi: 10.1093/toxsci/kfh140. [DOI] [PubMed] [Google Scholar]
- [148].Guerrero-Bosagna C, Savenkova M, Haque MM, Nilsoon E, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of altered Sertoli cell transcriptome and epigenome: molecular etiology of male infertility. PLos One. 2013;8:e59922. doi: 10.1371/journal.pone.0059922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Siu ER, Wong EW, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–44. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Su L, Mruk DD, Cheng CY. Regulation of drug transporters in the testis by environmental toxicant cadmium, steroids and cytokines. Spermatogenesis. 2012;2:285–93. doi: 10.4161/spmg.22536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 is critical to maintain the homeostasis of blood-testis barrier via its effects on tight junction reassembly. Proc Natl Acad Sci USA. 2010;107:17998–8003. doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109:12562–7. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Wan HT, Mruk DD, Li SYT, Mok KW, Lee WM, Wong CKC, et al. p-FAK-Tyr397 regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am J Physiol Endocrinol Metab. 2013;305:E687–E99. doi: 10.1152/ajpendo.00254.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12:1145–50. doi: 10.1016/s0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- [155].Albers J, Danzer C, Rechsteiner M, Lehmann H, Brandt LP, Hejhal T, et al. A versatile modular vector system for rapid combinatorial mammalian genetics. J Clin Invest. 2015;125:1603–19. doi: 10.1172/JCI79743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Schmid E, Neef S, Berlin C, Tomasovic A, Kahlert K, Nordbeck P, et al. Cardiac RKIP induces a beneficial beta-adrenoceptor-dependent positive inotropy. Nat Med. 2015;21:1298–306. doi: 10.1038/nm.3972. [DOI] [PubMed] [Google Scholar]
- [157].Tay TW, Andriana BB, Ishii M, Choi EK, Zhu XB, Alam MS, et al. An ultrastructural study on the effects of mono(2-ethylhexyl) phthalate on mice testes: cell death and sloughing of spermatogenic cells. Okajimas Folia Anat Jpn. 2007;83:123–30. doi: 10.2535/ofaj.83.123. [DOI] [PubMed] [Google Scholar]
- [158].Boekelheide K. Rat testis during 2,5-hexanedione intoxication and recovery. I. Dose response and the reversibility of germ cell loss. Toxicol Appl Pharmacol. 1988;92:18–27. doi: 10.1016/0041-008x(88)90223-2. [DOI] [PubMed] [Google Scholar]
- [159].Wang C, Fu W, Quan C, Yan M, Liu C, Qi S, et al. The role of Pten/Akt signaling pathway involved in BPA-induced apoptosis of rat Sertoli cells. Environ Toxicol. 2015;30:793–802. doi: 10.1002/tox.21958. [DOI] [PubMed] [Google Scholar]
- [160].Salian S, Doshi T, Vanage G. Neonatal exposure of male rats to Bisphenol A impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology. 2009;265:56–67. doi: 10.1016/j.tox.2009.09.012. [DOI] [PubMed] [Google Scholar]
- [161].Shi Y, Song Y, Wang Y, Liang X, Hu Y, Guan X, et al. p,p'-DDE induces apoptosis of rat Sertoli cells via a FasL-dependent pathway. J Biomed Biotechnol. 2009;2009:181282. doi: 10.1155/2009/181282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Song Y, Liang X, Hu Y, Wang Y, Yu H, Yang K. p,p'-DDE induces mitochondria-mediated apoptosis of cultured rat Sertoli cells. Toxicology. 2008;253:53–61. doi: 10.1016/j.tox.2008.08.013. [DOI] [PubMed] [Google Scholar]
- [163].Shi Y, Song Y, Wang Y, Liang X, Hu Y, Yu H, et al. beta-Benzene hexachloride induces apoptosis of rat Sertoli cells through generation of reactive oxygen species and activation of JNKs and FasL. Environ Toxicol. 2011;26:124–35. doi: 10.1002/tox.20536. [DOI] [PubMed] [Google Scholar]
- [164].Oh JH, Heo SH, Park HJ, Choi MS, Lee EH, Park SM, et al. Genomic and proteomic analyses of 1,3-dinitrobenzene-induced testicular toxicity in Sprague-Dawley rats. Reprod Toxicol. 2014;43:45–55. doi: 10.1016/j.reprotox.2013.10.004. [DOI] [PubMed] [Google Scholar]
- [165].Muguruma M, Yamazaki M, Okamura M, Moto M, Kashida Y, Mitsumori K. Molecular mechanism on the testicular toxicity of 1,3-dinitrobenzene in Sprague-Dawley rats: preliminary study. Arch Toxicol. 2005;79:729–36. doi: 10.1007/s00204-005-0006-8. [DOI] [PubMed] [Google Scholar]
- [166].Sonee M, Bogdan N, Hall L, Bryant S, Vinken P. The inhibin B response to the testicular toxicant 1, 3 dinitrobenzene in male rats. Birth Defects Res B Dev Reprod Toxicol. 2013;98:29–34. doi: 10.1002/bdrb.21038. [DOI] [PubMed] [Google Scholar]
- [167].Sobarzo CM, Lustig L, Ponzio R, Suescun MO, Denduchis B. Effects of di(2-ethylhexyl) phthalate on gap and tight junction protein expression in the testis of prepubertal rats. Microsc Res Tech. 2009;72:868–77. doi: 10.1002/jemt.20741. [DOI] [PubMed] [Google Scholar]
- [168].Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–9. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- [169].Hild SA, Reel JR, Larner JM, Blye RP. Disruption of spermatogenesis and Sertoli cell structure and function by the indenopyridine CDB-4022 in rats. Biol Reprod. 2001;65:1771–9. doi: 10.1095/biolreprod65.6.1771. [DOI] [PubMed] [Google Scholar]
- [170].Hild SA, Reel JR, Dykstra MJ, Mann PC, Marshall GR. Acute adverse effects of the indenopyridine CDB-4022 on the ultrastructure of sertoli cells, spermatocytes, and spermatids in rat testes: comparison to the known sertoli cell toxicant Di-n-pentylphthalate (DPP) J Androl. 2007;28:621–9. doi: 10.2164/jandrol.106.002295. [DOI] [PubMed] [Google Scholar]
- [171].Sod-Moriah UA, Sror U, Shemi D, Potashnik G, Chayoth R, Shaked I, et al. Long term effects of dibromochloropropane (DBCP) on male rats' reproductive system. Andrologia. 1988;20:60–6. doi: 10.1111/j.1439-0272.1988.tb02366.x. [DOI] [PubMed] [Google Scholar]
- [172].Meistrich ML, Wilson G, Shuttlesworth GA, Porter KL. Dibromochloropropane inhibits spermatogonial development in rats. Reprod Toxicol. 2003;17:263–71. doi: 10.1016/s0890-6238(03)00007-8. [DOI] [PubMed] [Google Scholar]
- [173].Potashnik G, Yanai-Inbar I, Sacks MI, Israeli R. Effect of dibromochloropropane on human testicular function. Isr J Med Sci. 1979;15:438–42. [PubMed] [Google Scholar]
- [174].Yang Y, Li F, Bi X, Sun L, Liu T, Jin Z, et al. Lead, zinc, and cadmium in vegetable/crops in a zinc smelting region and its potential human toxicity. Bull Environ Contam Toxicol. 2011;87:586–90. doi: 10.1007/s00128-011-0388-7. [DOI] [PubMed] [Google Scholar]
- [175].Barrett JR. Rice is a significant source of methylmercury: research in china assesses exposures. Environ Health Perspect. 2010;118:a398. doi: 10.1289/ehp.118-a398a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Ge RS, Chen GR, Tanrikut C, Hardy MP. Phthalate ester toxicity in Leydig cells: developmental timing and dosage considerations. Reprod Toxicol. 2007;23:366–73. doi: 10.1016/j.reprotox.2006.12.006. [DOI] [PubMed] [Google Scholar]
- [177].Guo Y, Zhang Z, Liu L, Li Y, Ren N, Kannan K. Occurrence and Profiles of Phthalates in Foodstuffs from China and Their Implications for Human Exposure. J Agric Food Chem. 2012 doi: 10.1021/jf3021128. [DOI] [PubMed] [Google Scholar]
- [178].Lie PP, Chan AY, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci U S A. 2010;107:11411–6. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Vauti F, Prochnow BR, Freese E, Ramasamy SK, Ruiz P, Arnold HH. Arp3 is required during preimplantation development of the mouse embryo. FEBS Lett. 2007;581:5691–7. doi: 10.1016/j.febslet.2007.11.031. [DOI] [PubMed] [Google Scholar]
- [180].Li N, Mruk DD, Wong CKC, Han D, Lee WM, Cheng CY. Formin 1 regulates ectoplamic specialization in the rat testis through its actin nucleation and bundling activity. Endocrinology. 2015;156:2969–83. doi: 10.1210/en.2015-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Hu J, Lu J, Lian G, Ferland RJ, Dettenhofer M, Sheen VL. Formin 1 and filamin B physically interact to coordinate chondrocyte proliferation and differentiation in the growht plate. Hum Mol Genet. 2014;23:4663–73. doi: 10.1093/hmg/ddu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Zhou F, Leder P, Zuniga a, Dettenbofer M. Formin 1 disruption confers oligodactyism and alters Bmp signaling. Hum Mol Genet. 2009;18:2472–82. doi: 10.1093/hmg/ddp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Offenhauser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, et al. Increased ethanol resistance and consumption in Eps8 knockouit mice correlates with altered actin dynamics. Cell. 2006;127:213–26. doi: 10.1016/j.cell.2006.09.011. [DOI] [PubMed] [Google Scholar]
- [184].Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, et al. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–3. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- [185].Tocchetti A, Soppo CB, Zani F, Bianchi F, Gagliani MC, Pozzi B, et al. Loss of the actin remodeler Eps8 causes intestinal defects and improved metabolic status in mice. PLoS One. 2010;5:e9468. doi: 10.1371/journal.pone.0009468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Tamura A, Kikuchi S, Hata M, Katsuno T, Matsui T, Hayashi H, et al. Achlorhydria by ezrin knockdown: defects in the formation/expansion of apical canaliculi in gastric parietal cells. J Cell Biol. 2005;169:21–8. doi: 10.1083/jcb.200410083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Yamakita Y, Matsumura F, Yamashiro S. Fascin 1 is dispensable for mouse development but is favorable for neonatal survival. Cell Motil Cytoskeleton. 2009;66:524–34. doi: 10.1002/cm.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Luo H, Liu X, Wang F, Huang Q, Shen S, Wang L, et al. Disruption of palladin results in neural tube closure defects in mice. Mol Cell Neurosci. 2005;29:507–15. doi: 10.1016/j.mcn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [189].van Dijk FS, Zillikens MC, Micha D, Riessland M, Marcelis CL, de Die-Smulders CE, et al. PLS3 mutations in X-linked osteoporosis with fractures. N Engl J Med. 2013;369:1529–36. doi: 10.1056/NEJMoa1308223. [DOI] [PubMed] [Google Scholar]
- [190].Berrueta L, Kraeft SK, Tirnauer JS, Schuyler SC, Chen LB, Hill DE, et al. The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc Natl Acad Sci U S A. 1998;95:10596–601. doi: 10.1073/pnas.95.18.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–30. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- [192].Su LK, Qi Y. Characterization of human MAPRE genes and their proteins. Genomics. 2001;71:142–9. doi: 10.1006/geno.2000.6428. [DOI] [PubMed] [Google Scholar]
- [193].Kita K, Wittmann T, Nathke IS, Waterman-Storer CM. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol Biol Cell. 2006;17:2331–45. doi: 10.1091/mbc.E05-06-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Straube A, Merdes A. EB3 regulates microtubule dynamics at the cell cortex and is required for myoblast elongation and fusion. Curr Biol. 2007;17:1318–25. doi: 10.1016/j.cub.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Diamantopoulos GS, Perez F, Goodson HV, Batelier G, Melki R, Kreis TE, et al. Dynamic localization of CLIP-170 to microtubule plus ends is coupled to microtubule assembly. J Cell Biol. 1999;144:99–112. doi: 10.1083/jcb.144.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Arnal I, Heichette C, Diamantopoulos GS, Chretien D. CLIP-170/tubulin-curved oligomers coassemble at microtubule ends and promote rescues. Curr Biol. 2004;14:2086–95. doi: 10.1016/j.cub.2004.11.055. [DOI] [PubMed] [Google Scholar]
- [197].Komarova YA, Akhmanova AS, Kojima S, Galjart N, Borisy GG. Cytoplasmic linker proteins promote microtubule rescue in vivo. J Cell Biol. 2002;159:589–99. doi: 10.1083/jcb.200208058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Weisbrich A, Honnappa S, Jaussi R, Okhrimenko O, Frey D, Jelesarov I, et al. Structure-function relationship of CAP-Gly domains. Nat Struct Mol Biol. 2007;14:959–67. doi: 10.1038/nsmb1291. [DOI] [PubMed] [Google Scholar]
- [199].Akhmanova A, Mausset-Bonnefont AL, van Cappellen W, Keijzer N, Hoogenraad CC, Stepanova T, et al. The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis. Genes Dev. 2005;19:2501–15. doi: 10.1101/gad.344505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009;138:366–76. doi: 10.1016/j.cell.2009.04.065. [DOI] [PubMed] [Google Scholar]
- [201].Leano JB, Rogers SL, Slep KC. A cryptic TOG domain with a distinct architecture underlies CLASP-dependent bipolar spindle formation. Structure. 2013;21:939–50. doi: 10.1016/j.str.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Schmidt N, Basu S, Sladecek S, Gatti S, van Haren J, Treves S, et al. Agrin regulates CLASP2-mediated capture of microtubules at the neuromuscular junction synaptic membrane. J Cell Biol. 2012;198:421–37. doi: 10.1083/jcb.201111130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, et al. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol. 2005;168:141–53. doi: 10.1083/jcb.200405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Mimori-Kiyosue Y, Shiina N, Tsukita S. Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J Cell Biol. 2000;148:505–18. doi: 10.1083/jcb.148.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Munemitsu S, Souza B, Muller O, Albert I, Rubinfeld B, Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–81. [PubMed] [Google Scholar]
- [206].Bienz M. The subcellular destinations of APC proteins. Nat Rev Mol Cell Biol. 2002;3:328–38. doi: 10.1038/nrm806. [DOI] [PubMed] [Google Scholar]
- [207].Honnappa S, John CM, Kostrewa D, Winkler FK, Steinmetz MO. Structural insights into the EB1-APC interaction. EMBO J. 2005;24:261–9. doi: 10.1038/sj.emboj.7600529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Wang X, Imura T, Sofroniew MV, Fushiki S. Loss of adenomatous polyposis coli in Bergmann glia disrupts their unique architecture and leads to cell nonautonomous neurodegeneration of cerebellar Purkinje neurons. Glia. 2011;59:857–68. doi: 10.1002/glia.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, et al. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Gard DL, Kirschner MW. A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J Cell Biol. 1987;105:2203–15. doi: 10.1083/jcb.105.5.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Vasquez RJ, Gard DL, Cassimeris L. XMAP from Xenopus eggs promotes rapid plus end assembly of microtubules and rapid microtubule polymer turnover. J Cell Biol. 1994;127:985–93. doi: 10.1083/jcb.127.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Barbarese E, Ifrim MF, Hsieh L, Guo C, Tatavarty V, Maggipinto MJ, et al. Conditional knockout of tumor overexpressed gene in mouse neurons affects RNA granule assembly, granule translation, LTP and short term habituation. PLoS One. 2013;8:e69989. doi: 10.1371/journal.pone.0069989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Goodwin SS, Vale RD. Patronin regulates the microtubule network by protecting microtubule minus ends. Cell. 2010;143:263–74. doi: 10.1016/j.cell.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Hendershott MC, Vale RD. Regulation of microtubule minus-end dynamics by CAMSAPs and Patronin. Proc Natl Acad Sci U S A. 2014;111:5860–5. doi: 10.1073/pnas.1404133111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Baines AJ, Bignone PA, King MD, Maggs AM, Bennett PM, Pinder JC, et al. The CKK domain (DUF1781) binds microtubules and defines the CAMSAP/ssp4 family of animal proteins. Mol Biol Evol. 2009;26:2005–14. doi: 10.1093/molbev/msp115. [DOI] [PubMed] [Google Scholar]
- [216].Chuang M, Goncharov A, Wang S, Oegema K, Jin Y, Chisholm AD. The microtubule minus-end-binding protein patronin/PTRN-1 is required for axon regeneration in C. elegans. Cell Rep. 2014;9:874–83. doi: 10.1016/j.celrep.2014.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Zhang D, Rogers GC, Buster DW, Sharp DJ. Three microtubule severing enzymes contribute to the "Pacman-flux" machinery that moves chromosomes. J Cell Biol. 2007;177:231–42. doi: 10.1083/jcb.200612011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Yu W, Solowska JM, Qiang L, Karabay A, Baird D, Baas PW. Regulation of microtubule severing by katanin subunits during neuronal development. J Neurosci. 2005;25:5573–83. doi: 10.1523/JNEUROSCI.0834-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Hartman JJ, Vale RD. Microtubule disassembly by ATP-dependent oligomerization of the AAA enzyme katanin. Science. 1999;286:782–5. doi: 10.1126/science.286.5440.782. [DOI] [PubMed] [Google Scholar]
- [220].Quarmby L. Cellular Samurai: katanin and the severing of microtubules. J Cell Sci. 2000;113:2821–7. doi: 10.1242/jcs.113.16.2821. Pt 16. [DOI] [PubMed] [Google Scholar]
- [221].Mains PE, Kemphues KJ, Sprunger SA, Sulston IA, Wood WB. Mutations affecting the meiotic and mitotic divisions of the early Caenorhabditis elegans embryo. Genetics. 1990;126:593–605. doi: 10.1093/genetics/126.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Errico A, Ballabio A, Rugarli EI. Spastin, the protein mutated in autosomal dominant hereditary spastic paraplegia, is involved in microtubule dynamics. Hum Mol Genet. 2002;11:153–63. doi: 10.1093/hmg/11.2.153. [DOI] [PubMed] [Google Scholar]
- [223].Roll-Mecak A, Vale RD. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature. 2008;451:363–7. doi: 10.1038/nature06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Tarrade A, Fassier C, Courageot S, Charvin D, Vitte J, Peris L, et al. A mutation of spastin is responsible for swellings and impairment of transport in a region of axon characterized by changes in microtubule composition. Hum Mol Genet. 2006;15:3544–58. doi: 10.1093/hmg/ddl431. [DOI] [PubMed] [Google Scholar]
- [225].Hazan J, Fonknechten N, Mavel D, Paternotte C, Samson D, Artiguenave F, et al. Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat Genet. 1999;23:296–303. doi: 10.1038/15472. [DOI] [PubMed] [Google Scholar]
- [226].Mukherjee S, Diaz Valencia JD, Stewman S, Metz J, Monnier S, Rath U, et al. Human Fidgetin is a microtubule severing the enzyme and minus-end depolymerase that regulates mitosis. Cell Cycle. 2012;11:2359–66. doi: 10.4161/cc.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci U S A. 2001;98:7004–11. doi: 10.1073/pnas.111145398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Vale RD, Fletterick RJ. The design plan of kinesin motors. Annu Rev Cell Dev Biol. 1997;13:745–77. doi: 10.1146/annurev.cellbio.13.1.745. [DOI] [PubMed] [Google Scholar]
- [229].Varga V, Helenius J, Tanaka K, Hyman AA, Tanaka TU, Howard J. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nat Cell Biol. 2006;8:957–62. doi: 10.1038/ncb1462. [DOI] [PubMed] [Google Scholar]
- [230].Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- [231].Helenius J, Brouhard G, Kalaidzidis Y, Diez S, Howard J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature. 2006;441:115–9. doi: 10.1038/nature04736. [DOI] [PubMed] [Google Scholar]
- [232].Zhao C, Takita J, Tanaka Y, Setou M, Nakagawa T, Takeda S, et al. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 2001;105:587–97. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]
- [233].Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–26. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- [234].Oiwa K, Sakakibara H. Recent progress in dynein structure and mechanism. Curr Opin Cell Biol. 2005;17:98–103. doi: 10.1016/j.ceb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [235].Afzelius BA, Eliasson R. Male and female infertility problems in the immotile-cilia syndrome. Eur J Respir Dis Suppl. 1983;127:144–7. [PubMed] [Google Scholar]
- [236].Saaranen M, Vierula M. [Immotile cilia syndrome as a cause of male infertility] Duodecim. 1984;100:1121–5. [PubMed] [Google Scholar]