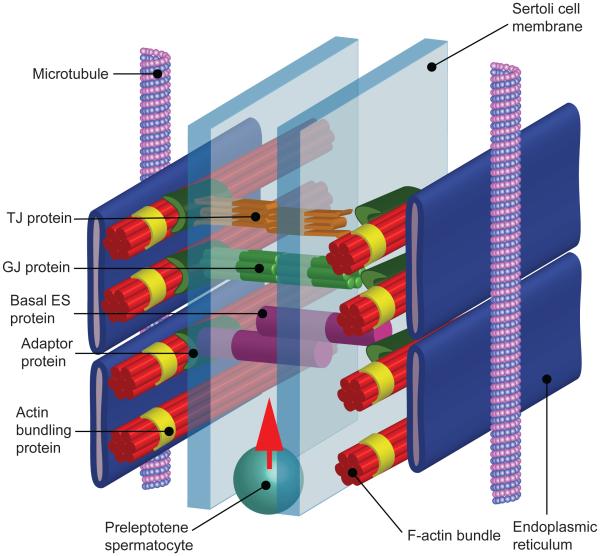
Figure 1. A schematic drawing illustrating the ulstrastructural features of actin-based adhesion proteins that constitute the blood-testis barrier (BTB) in rodents and humans.
The most noticeable structural feature of the BTB, constituted by tight junction (TJ), basal ectoplasmic specialization (basal ES), and gap junction (GJ) between adjacent Sertoli cells located near the basement membrane of the seminiferous epithelium, is the presence of an array of actin microfilament bundles that are sandwiched between cisternae of endoplasmic reticulum (ER) and apposing Sertoli cel plasma membranes. The BTB is located above the preleptotene spermatocytes which are set to be transported across the BTB at stage VIII of the epithelial cycle. As shown herein, adhesion protein complexes of the TJ (e.g., occludin-ZO-1), basal ES (e.g., N-cadherin-β-catenin), and GJ (e.g., connexin43-plakophilin-2) (note: occludin, N-cahderin, and connexin 43 are the integral membrane proteins of the TJ, basal ES and GJ, respectively, whereas ZO-1, β-catenin, and plakophilin-2 are the corresponding adaptor protein of TJ, basal ES and GJ) are anchored onto the actin microfilaments via the corresponding adpator protein. Actin microfilament bundles are maintained via the action of actin bundling proteins such as Eps8, pallain, and plastins. Microtubules (MT) also also located near the actin microfilaments which serve as the tracks for the transport of preleptotene spermatocytes across the BTB at stage VIII of the epithelial cycle.