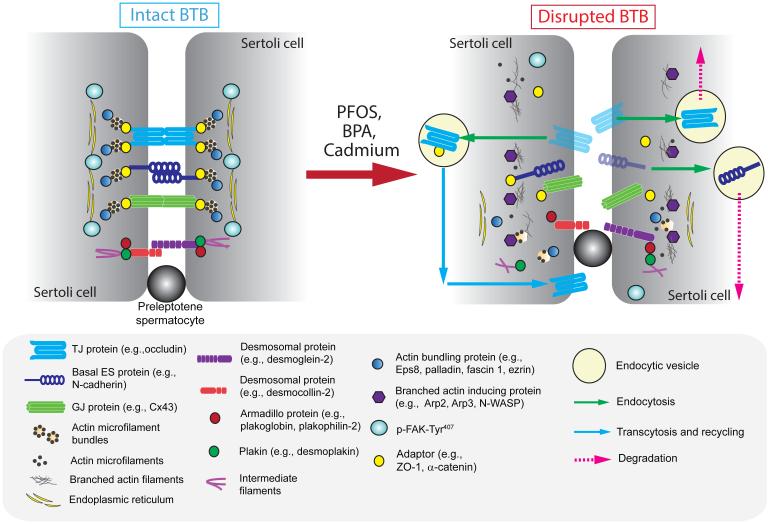
Figure 3. A molecular model by which environmental toxicants induce Sertoli and testicular injury via its initial effects at the BTB.
The left panel illustrates the detailed molecular view of an intact BTB, such as at stage VII of the epithelial cycle in the rat testis, which is conferred by the adhesion protein complexes of the actin-based TJ, basal ES and GJ, as well as the intermediate filament based desmosome proteins between adjacent Sertoli cells. As shown in Figure 1, one of the most typical features of the BTB is the prominent presence of actin microfilaments that aligned perpendicular to the Sertoli cell plasma membrane and are sandwiched in-between the cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane. This array of actin microfilament bundles supported by the enhanced expression of actin bundling proteins (e.g., Eps8 also barbed end capping protein, palladin, plastin 3, fascin 1) and p-FAK-Tyr407, and a reduced expression of branched actin polymerization protein Arp3, also confer the strong adhesive strength of the BTB, making it one of the tighest blood-tessue barriers in the mammalian body. However, exposure of the Sertoli cells isolated from either rodents or humans to the environmental toxicants, such as PFOS, BPA and cadmium as discussed in text, with an established TJ-permeability barrier that mimics the BTB in vivo, these toxicants rapidly alter the spatiotemporal expression of two major groups of actin binding proteins (APBs), such as (i) actin barbed end capping and bundling protein Eps8 or other actin bundling/cross-linking proteins (e.g., palladin, plastin 3, fascin 1, ezrin) that confer actin microfilaments their bundled configuration, and (ii) branched acin polymerization protein Arp3. In short, a reduced expression of actin bundling proteins and p-FAK-Tyr407 are noted, concomitant with an up-regulation of Arp3 at nor near the Sertoli cell-cell interface. The net result in turn destabilizes adhesion protein complexes are the site, either undergo endosome-mediated protein degradation or transcytosis and recycling. This thus facilitates the entry of more environmental toxicants to the adluminal compartment, perturbing either meiosis I/II or post-meiotic spermatid development, spermatid transport and spermatid polarity.