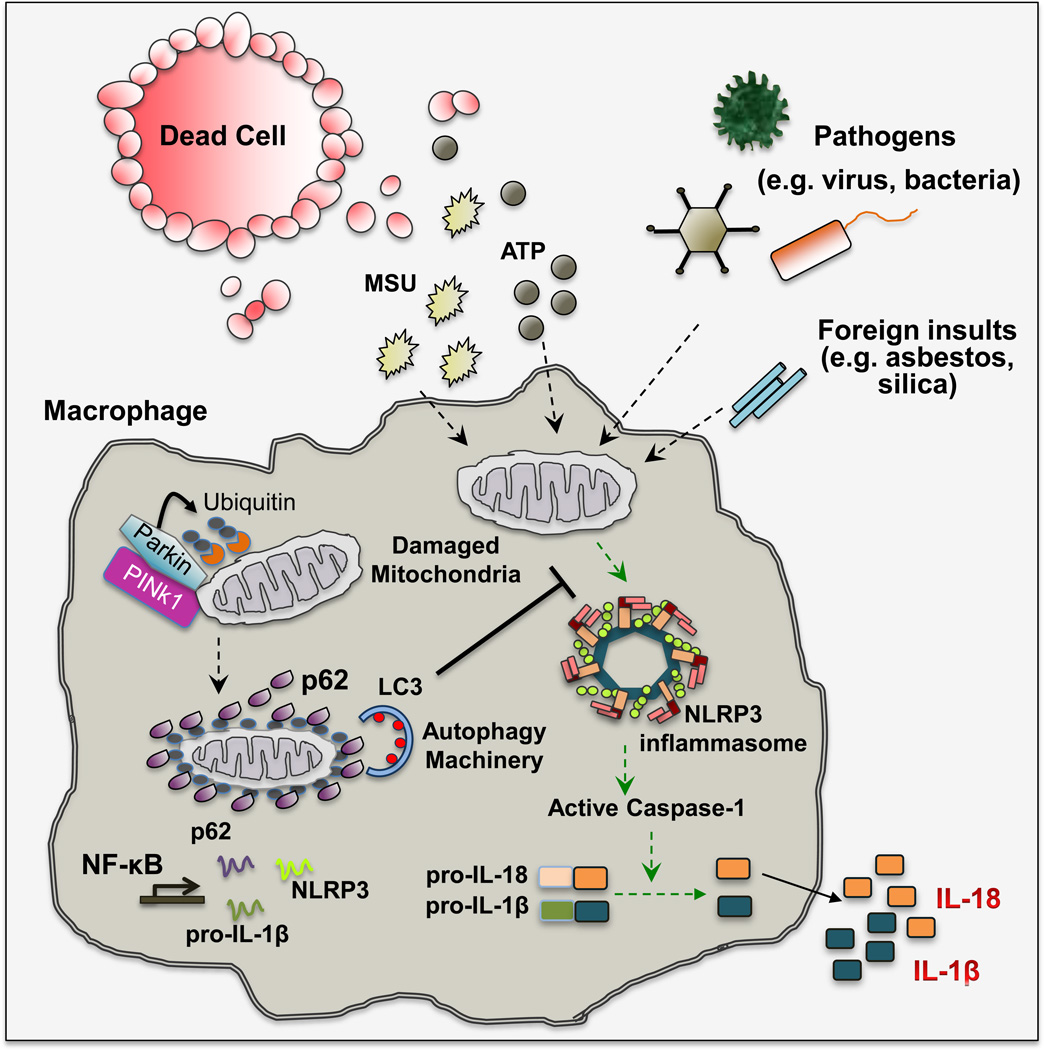
Figure 3. Mitophagy/Autophagy Dials Down Inflammation.
Stimulation of macrophages with DAMP (e.g. ATP and uric acid), carcinogenic particles (asbestos and silica microfibers/crystals) or PAMP (e.g. bacterial toxins) results in mitochondrial damage that is characterized by loss of mitochondrial membrane potential and subsequent release of mtDNA and mtROS. These mitochondrial signals in turn activate the NLRP3 inflammasome to induce IL-1β and IL-18 secretion. Loss of mitochondrial membrane potential (ψm) activates PINK1, a mitochondrial protein kinase that phosphorylates ubiquitin chains attached to mitochondrial outer membrane proteins. Phosphorylated ubiquitin interacts with and activates Parkin, an E3 ubiquitin ligase that further ubiquitinates mitochondrial outer membrane proteins. Ubiquitin-tagged mitochondria are recognized by the UBA domain of p62, whose expression is induced upon NF-κB activation. p62 also binds to LC3 and targets ubiquitinated mitochondria to autophagosomal clearance. By eliminating signal-emitting mitochondria, macrophage limits the extent of NLRP3-inflammasome activation. MSU, monosodium urate crystal; PINK1, PTEN-induced putative kinase 1; LC3, microtubule-associated protein 1A/1B light chain 3; NF-κB, nuclear factor-kappa B; UBA, ubiquitin association domain.