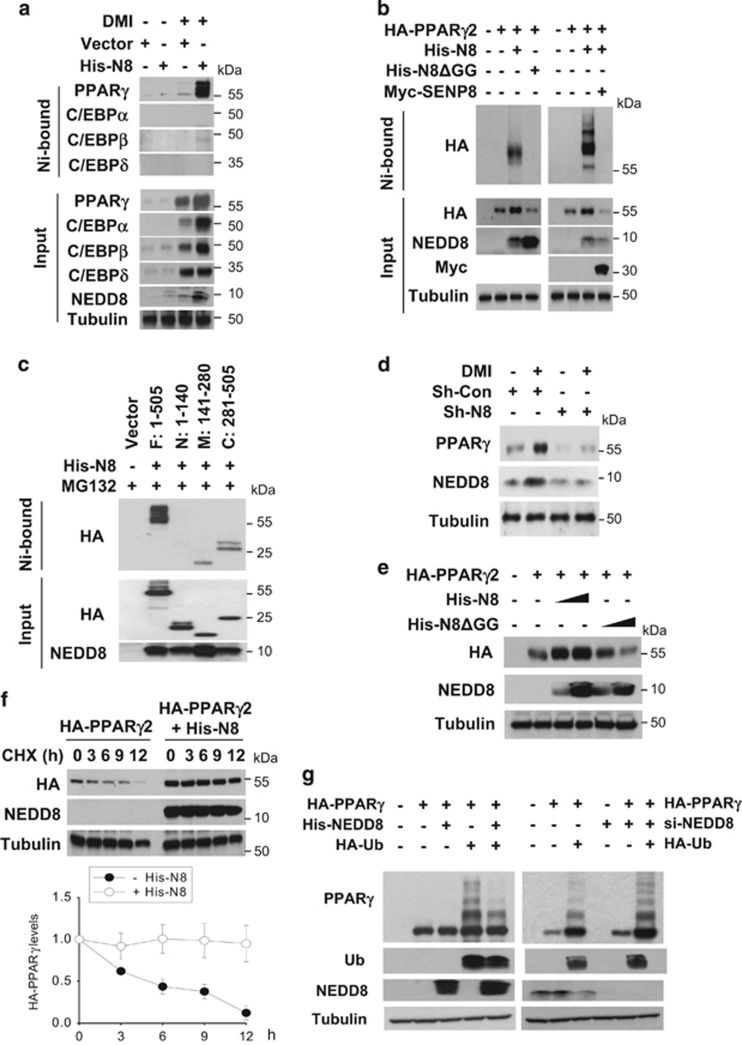
Figure 3.
Neddylated PPARγ is stabilized by inhibition of its ubiquitination. (a) PPARγ is conjugated with NEDD8. 3T3-L1 cells stably expressing pLVX-IRES or pLVX-IRES-His-NEDD8 were differentiated with DMI and subjected to western blotting after pull-down purification using a Ni2+ column under denaturing conditions. (b) Ectopically expressed PPARγ2 is neddylated. HEK293 cells were cotransfected with pHA-PPARγ2, pHis-NEDE8, pHis-NEDD8ΔGG, and pMyc-SENP8 plasmids in the indicated combinations. Proteins isolated using Ni2+ were analyzed by western blotting. (c) Identification of neddylated domains of PPARγ. HEK293 cells were cotransfected with pHis-NEDE8 and one of four plasmids for HA-PPARγ2 fragments. PPARγ2 fragments were isolated using Ni2+ and then analyzed by western blotting. The amino acids of expressed peptides are indicated at the top panel. (d) NEDD8 is required for PPARγ expression. 3T3-L1 cells stably expressing sh-control (Sh-Con) or shNEDD8 (Sh-N8) were stimulated with DMI, and then cell lysates were subjected to western blotting. (e) NEDD8 promotes the expression of ectopic PPARγ. HEK293 cells were cotransfected with pHA- PPARγ2 and pHis-NEDD8 or pHis-NEDD8ΔGG, and then cell lysates were analyzed by western blotting using an anti-HA antibody. (f) PPARγ is stabilized by NEDD8. HEK293 cells were transfected with pHA-PPARγ2 and/or pHis-NEDE8, and then incubated with cycloheximide for the indicated time. Cell lysates were subjected to western blotting using anti-HA or anti-NEDD8 antibody (upper panel). Band intensities (mean±S.D., n=3) on blots were analyzed using ImageJ 1.36b and plotted (lower panel). (g) Neddylation competes with ubiquitination in PPARγ. HEK293 cells were cotransfected with the indicated plasmids or siRNAs. Then they were stabilized for 48 h. After incubated with 10 μM MG132 for 8 h, cells were subjected to immunoblot analyses. β-Tubulin was determined as a loading control