Figure 4.
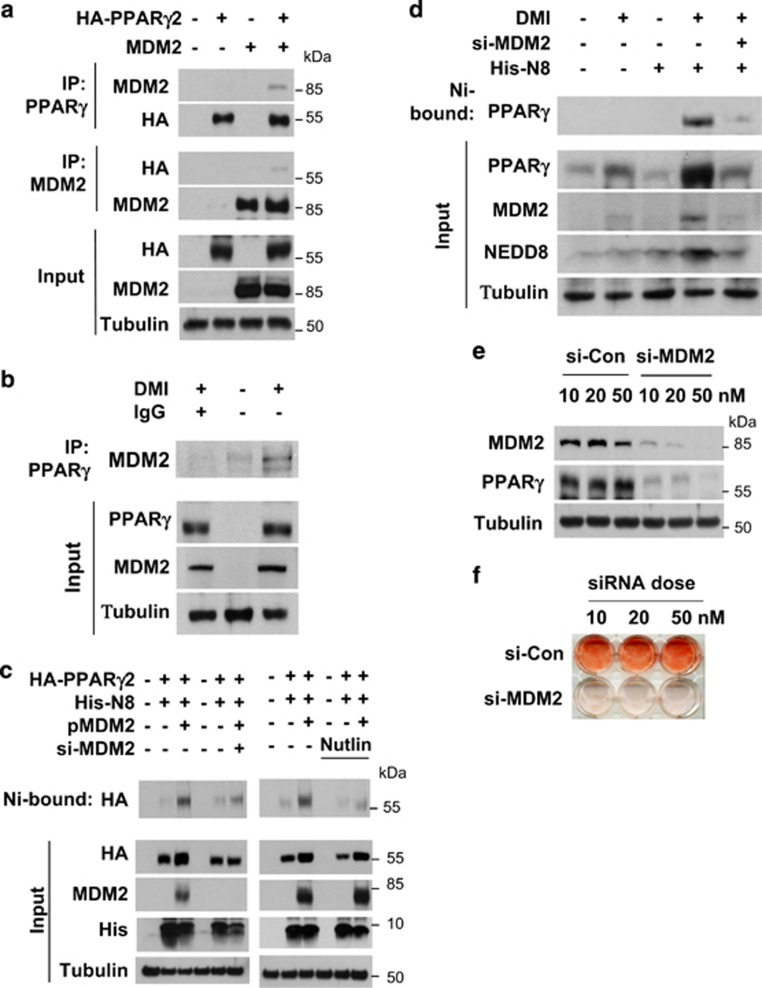
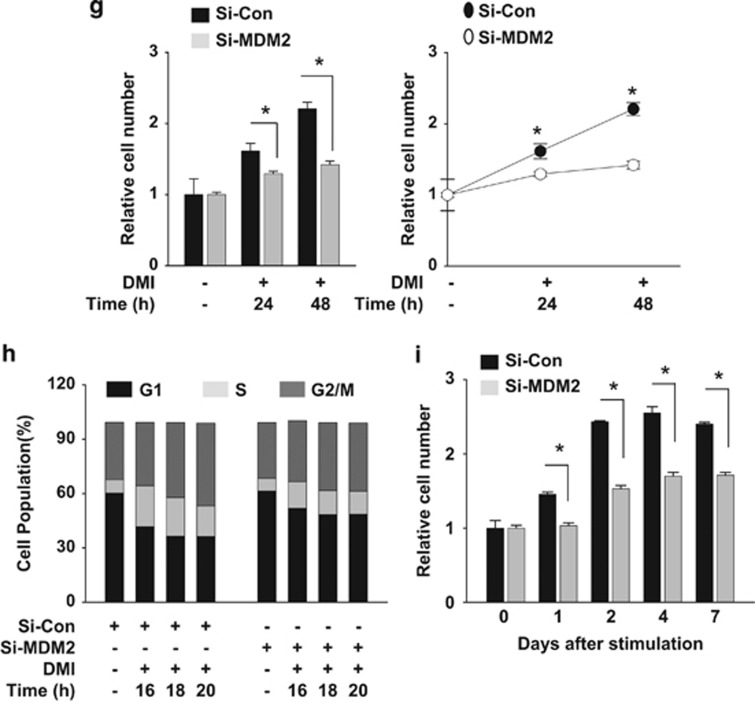
PPARγ neddylation is mediated by MDM2. (a) Ectopically expressed MDM2 interacts with PPARγ. HEK293 cells were cotransfected with pHA-PPARγ2 and pcMDM2. Proteins in cell lysates were immunoprecipitated by anti-PPARγ or anti-MDM2 antibody, and immunoprecipitates were analyzed by western blotting. (b) Endogenous MDM2 interacts with PPARγ. 3T3-L1 cells were differentiated by DMI for 2 days, and then cell lysates were subjected to immunoprecipitation with PPARγ antibody. Immunoprecipitates were analyzed by western blotting. (c) Ectopic MDM2 induces the neddylation of PPARγ. HEK293 cells were cotransfected with pHA-PPARγ2, pHis-NEDE8, pcMDM2, and/or si-MDM2. His-NEDD8-conjugated PPARγ was purified using a Ni2+ affinity column and analyzed by western blotting. Protein expressions (Input) were examined to verify transfection efficiency. (d) MDM2 neddylates PPARγ endogenously. 3T3-L1 cells were cotransfected with the NEDD8 plasmid and si-Control or si-MDM2. Transfected cells were treated with DMI to stimulate adipogenesis, and then lysed in a denaturing condition. Neddylated PPARγ was pulled-down with Ni2+ affinity resin, and then analyzed by immunoblotting. β-Tubulin was used as an input control. (e) MDM2 is required for PPARγ expression. 3T3-L1 cells were transfected with si-Control or si-MDM2, and then cell lysates were subjected to western blotting for checking MDM2 or PPARγ expression. (f) MDM2 is required for lipid storage in adipocytes. 3T3-L1 cells, which had been transfected with si-control or si-MDM2 at the indicated concentrations, were differentiated with DMI for 8 days and subjected to Oil Red O staining. (g) Knockdown of MDM2 partially blocks clonal expansion in 3T3-L1 preadipocytes. 3T3-L1 cells, which had been transfected with non-targeting or MDM2-targeting siRNAs, were cultured to reach confluence, and the cells were further cultured for two days. Cells were treated with DMI and incubated for the indicated times. Cell numbers were counted using a hemocytometer and presented relatively to that on day 0. Each bar represents the mean+S.D. from three independent experiments. *denotes P<0.05. (h) Two-day post-confluent 3T3-L1 preadipocytes, which had been transfected with siRNAs, were incubated with DMI. On the indicated times, cells were stained with propidium iodide and subjected to flow cytometry to analyze cell cycle. The data are presented as the mean values from three independent experiments