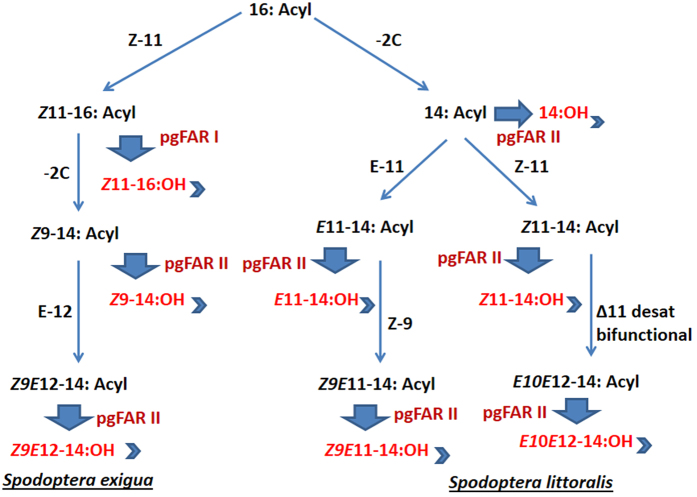
Figure 8. Spodoptera exigua and S. littoralis pheromone biosynthesis pathway for the production of pheromone precursors.
The de novo biosynthesis of all precursors starts from palmitoyl-CoA (16:acyl). In S. exigua, a Δ11 desaturase catalyzes the production of (Z)-11-hexadecenyl [(Z)-11–16:acyl], which undergoes one cycle of β-oxidation to produce (Z)-9-tetradecenyl [(Z)-9–14:acyl]22. A Δ12 desaturase catalyses (Z)-9–14:acyl to produce (Z,E)-9,12- tetradecadienyl acyl (Z9E12-14:acyl). In S. littoralis, 16:acyl undergoes one cycle of β-oxidation to produce myristoyl-CoA (14:acyl). A Δ11 desaturase acts on 14:Acyl to produce (E)- and (Z)-11-tetradecenoyl [(E) + (Z)-11–14:acyl]21. A Δ9 desaturase acts on (E)-11–14:acyl to produce (Z,E)-9,11- tetradecadienyl acyl (Z9E11-14:acyl), and a bifunctional Δ11 desaturase acts on (Z)-11–14:acyl to produce (E,E)-10,12- tetradecadienyl acyl (E10E12-14:acyl)50. Each compound undergoes a reduction step catalysed by pgFAR I and pgFAR II to produce the corresponding fatty alcohol derivatives. Finally, an acetyltransferase catalyses the conversion of the fatty alcohol precursors into the corresponding acetate esters (shown with an arrowhead).