Abstract
Identification of biomarkers that assess post-transplant risk is needed to improve long-term outcomes following heart transplantation. The Clinical Trials in Organ Transplantation (CTOT)-05 protocol was an observational, multicenter, cohort study of 200 heart transplant recipients followed for the first post-transplant year. The primary endpoint was a composite of death, graft loss/re-transplantation, biopsy proven acute rejection (BPAR) and cardiac allograft vasculopathy (CAV) as defined by intravascular ultrasound (IVUS). We serially measured anti-HLA- and auto-antibodies, angiogenic proteins, peripheral blood allo-reactivity and peripheral blood gene expression patterns. We correlated assay results and clinical characteristics with the composite endpoint and its components. The composite endpoint was associated with older donor allografts (p<0.03) and with recipient anti-HLA antibody (p<0.04). Recipient CMV-negativity (regardless of donor status) was associated with BPAR p<0.001), and increases in plasma vascular endothelial growth factor-C (OR 20; 95%CI:1.9–218) combined with decreases in endothelin-1: (OR 0.14; 95%CI:0.02–0.97) associated with CAV. The remaining biomarkers showed no relationships with the study endpoints. While suboptimal endpoint definitions and lower than anticipated event rates were identified as potential study limitations, the results of this multicenter study do not yet support routine use of the selected assays as noninvasive approaches to detect BPAR and/or CAV following heart transplantation.
Introduction
Identification and validation of accurate and reproducible, noninvasive biomarkers capable of diagnosing and/or predicting outcomes following heart transplantation has the potential to improve clinical care and patient health. Validated biomarkers for incipient acute rejection (AR) could diminish biopsy-related morbidity and guide decision-making that optimizes immune suppressant dosing, thereby limiting side effects and preventing development of irreversible allograft damage. AR-related morbidity following heart transplantation remains significant (1, 2), supporting the need for predictive biomarkers capable of detecting this endpoint. Median survival of heart allografts remains suboptimal at ~11 years (1, 2), underscoring the pressing need for biomarkers of late outcomes. Cardiac allograft vasculopathy (CAV), a manifestation of chronic injury, is commonly associated with graft deterioration and failure and there are no available therapies capable of reversing CAV once it has been initiated. Thus, identifying and validating markers of early or incipient CAV could be transformative and would support future clinical trials in which preventative interventions could be tested for their ability to improve allograft and patient survival.
With the exception of one multicenter study showing that a peripheral blood gene profile can bypass performing allograft biopsies to detect acute rejection at times >6-months post-transplantation (3), reports of biomarkers in heart transplant recipients have been relatively small, cross-sectional, single center analyses and few have identified predictive biomarkers for CAV at early times post-transplantation (4–20). Several studies have provided evidence that alloreactive T cells detected in peripheral blood (21–26), the quantity of cell-free, donor DNA in recipient’s plasma (27, 28), serum angiogenesis-related factors (29–33), serum anti-HLA antibodies and autoantibodies (34–39) and several peripheral blood gene profiles are associated with acute or chronic heart graft injury (40–43). Prospective, multicenter, comparative analyses of candidate biomarkers for AR and CAV have not been reported. How biomarkers relate to known clinical risk factors associated with these endpoints in heart transplant recipients are also not known.
In an effort to address these deficiencies, we designed the Clinical Trials in Organ Transplantation-05 (CTOT-05) trial, a multicenter, observational, study correlating biomarkers with outcomes in first heart transplant recipients. We chose to study a panel of candidate peripheral blood cell biomarkers that were deemed potentially informative based on published single center studies from the heart transplant and/or the kidney transplant literature. We serially collected peripheral blood and biopsy samples over the first year following heart transplantation and assessed independent relationships of the biomarkers with a composite endpoint comprised of graft loss, incidence of rejection and presence of CAV at 12-months, as well as with each of its components.
Methods
Study design and oversight
This prospective multicenter observational trial (clinicaltrials.gov NCT00466804) had a target accrual of 200 adult recipients of primary heart transplantation. The CTOT-05 protocol development team was led by P. Heeger, M. Sayegh and R. Starling. Medical safety oversight was provided by N. Bridges. Statistical analysis was the responsibility of D. Ikle (with the CTOT-05 team). Data were collected by the investigators and coordinators at each site. All authors are responsible for data accuracy and completeness. Each site participated under the auspices of its Institutional Review Board. An independent, NIAID-appointed Data Safety Monitoring Board was responsible for periodic safety review.
Subjects
Adult candidates for heart transplantation were eligible for enrollment. Detailed inclusion and exclusion criteria are shown in Table S1.
Endpoints
The primary endpoint was a composite of death, re-transplantation/re-listing, biopsy proven acute rejection (BPAR), and the incidence of rapidly progressive CAV defined as an incremental change in IVUS-measured coronary artery maximal intimal thickness (MIT) of >0.5 mm from 6–8 weeks post-transplant to 12 months post-transplant in a matched site (44). Secondary endpoints included each component of the composite endpoint.
Central pathology readings of tissue sections were performed blinded by J. Stone (MGH) according to the ISHLT 2005 working formulation (45). We defined BPAR as acute cellular rejection ISHLT >grade 2R. Hemodynamic compromise was not analyzed as an endpoint due to lack of objective evidence to adjudicate. Biopsies were read locally for clinical management. Tissue from the same biopsy (for some centers, different sections) was submitted to and read by the core pathology laboratory.
IVUS was performed at each site using a standardized protocol developed at the Cleveland Clinic (46, 47). Recordings were sent to the central IVUS reading laboratory (M Tuzcu and S Nicholls) where they were assessed by standard quality assurance (QA) criteria (44, 48, 49). If either IVUS reading from an individual subject was not obtained or did not meet QA criteria the subject was deemed ineligible for evaluation of the IVUS endpoint. Subjects who did not meet the CAV endpoint could still meet the composite endpoint if they died, were re-listed, or developed BPAR.
Interventions and sample collection
Immunosuppression was not standardized; doses and levels of immunosuppressive drugs were defined and maintained within therapeutic ranges as per local practice (Figure S1). Standard of care surveillance endomyocardial biopsies were obtained at weeks 2 and 6 and months 2, 3, 4, 5, 6, 9, and 12 post-transplantation. “For-cause” biopsies were obtained per local center practice.
Blood samples were obtained prior to transplantation, at week 6 and months 3, 6, 9, and 12 post-transplant. Study visits occurred whenever a clinically indicated biopsy was scheduled. Blood samples were collected prior to biopsies or associated treatments.
Surveillance studies for cytomegalovirus (CMV) were performed according to local practice at each participating site. Prophylaxis against CMV and Pneumocystis jirovecii was per local standard of care.
Laboratory studies
Anti-HLA antibody analysis
Anti-HLA antibodies were measured at the core laboratory at the Brigham and Women’s Hospital (I. Guleria) using Luminex LABScreen® Single Antigen HLA Class I and Class II Antibody Detection. Assignment of a DSA required a median fluorescent intensity >1000 (a predetermined albeit relatively low threshold chosen to minimize the false negative rate) and an appropriate donor-specific epitope pattern following review of recipient and donor HLA types and the anti-HLA specificities.
ELISPOT Panel of Reactive T cell (PRT) assays
PBMCs obtained at baseline were stimulated against a panel of 6 allogeneic B cell lines in IFNγ ELISPOT assays, in triplicate as described previously in detail (50, 51). Mean values for responses to each stimulator were summed to derive the PRT value.
Plasma angiogenesis-related proteins
Plasma collected at the time of transplant and 1-year post-transplant was stored at −80°C. Concentrations of 17 angiogenesis-related proteins were initially measured in batches using a multiplex magnetic bead based assay (Millipore, Billerica, MA) on a LX 200 analyzer (Luminex, Austin, TX), according to the manufacturer’s instructions. Initially, 20 subjects who met the CAV endpoint and 40 controls were randomly selected from the 106 subjects with paired IVUS evaluations. Based on these results, ELISAs for VEGF-A, VEGF-C, Leptin, and Endothelin-1 (ET-1) (R&D Systems; Minneapolis, MN) were performed on all available baseline and 1-year post- transplant samples collected from the 106 subjects with evaluable paired IVUS results.
Peripheral blood and tissue gene expression profiling
Biopsy samples were immediately placed in 150µl RNAlater (Life Technologies, Grand Island, NY) and stored at −70°C. After thawing, samples were homogenized using Tissuelyser (Qiagen, Hilden, Germany), and total RNA was extracted using Purelink micro to midi Total RNA Purification System (Invitrogen, Carlsbad, CA). Peripheral blood was collected in PAXgene RNA collection tubes (BD Diagnostics, Valencia, CA), stored at room temperature for 6–24 hours, then frozen at −70°C. Total RNA was by PAXgene blood miRNA kit (PreAnalytiX, QIAGEN, Hilden, Germany). 1µg of total RNA/100 µl was converted into complementary DNA (cDNA) using Taqman Reverse Transcription kit (Applied Biosystems/Life Technologies, Foster City, CA, USA).
Real-time PCR (RT-qPCR) analysis was performed by a two-step process—a 10-cycle preamplification step (AmpliTaq® DNA Polymerase Kit; Applied Biosystems/Life Technologies, Foster City, CA, USA) followed by measurement of mRNA copies with an ABI PRISM 7900HT Sequence Detection System (for details and primers see supplemental methods).
Statistical Methods
Original sample size calculations were based on the reported incidence of anti-HLA antibodies and their relationship with CAV. A two-sided Chi-square test at the alpha level of 0.05 was expected to achieve 80% power to detect odd ratios in the range of 2.7 to 3.5 (comparing presence of CAV to absence of CAV) with a sample size of 150.
Data are summarized using descriptive statistics for categorical (counts/percentages) and continuous (mean and standard deviations) variables. Univariate analyses were performed using chi-square, Fisher’s Exact, or Cochran-Mantel-Haenszel tests for categorical variables and t-tests for continuous variables. Log10 transformations applied as necessary to satisfy normal distribution assumptions. Univariate and multivariable logistic regression methods were used to model the relationships between markers and endpoints of interest. See the Supplemental Methods section for greater detail. All statistical analyses were performed in SAS Version 9.3 (SAS, Cary, NC).
Results
Description of cohort
We enrolled 263 heart transplant candidates at 12 sites in the US between 2007 and 2010 (Table 1). The final study cohort was composed of the first 200 subjects who underwent heart transplantation. This cohort was predominantly Caucasian (74.5%) and male (81%) with a mean age of 54 years. 43% were CMV IgG-negative (regardless of donor serology) and 36% were supported by left ventricular assist devices (LVAD) at the time of transplantation. Organ donors had a mean age of 31 years, 67.5% were Caucasian, and 39.5% were CMV-antibody negative.
Table 1.
Baseline characteristics for all 200 transplanted subjects
| Characteristics | Total Transplanted (N=200) |
|---|---|
| Donor Characteristics | |
| Age | |
| Mean ± SD | 31.1 ± 11.84 |
| Male Gender | 139 (69.5) |
| Race | |
| White | 135 (67.5) |
| Black or African American | 34 (17.0) |
| Other | 6 ( 3.0) |
| Unknown or Not Reported | 25 (12.5) |
| Ethnicity | |
| Hispanic or Latino | 33 (16.5) |
| Not Hispanic or Latino | 141 (70.5) |
| Unknown or Not Reported | 26 (13.0) |
| Cause of Death | |
| Anoxia | 31 (15.5) |
| Cerebrovascular/Stroke | 27 (13.5) |
| Head Trauma | 102 (51.0) |
| Other | 40 (20.0) |
| CMV IgG Status [1] | |
| Positive | 118 (59.0) |
| Negative | 79 (39.5) |
| Recipient Characteristics | |
| Age (year) | |
| Mean ± SD | 53.6 ± 12.40 |
| Male Gender | 162 (81.0) |
| Race | |
| White | 149 (74.5) |
| Black or African American | 32 (16.0) |
| Other | 9 ( 4.5) |
| Unknown or Not Reported | 10 ( 5.0) |
| Ethnicity | |
| Hispanic or Latino | 14 ( 7.0) |
| Not Hispanic or Latino | 173 (86.5) |
| Unknown or Not Reported | 13 ( 6.5) |
| Weight (kg) (N=188) | |
| Mean ± SD | 81.6 ± 15.63 |
| BMI (kg/m²) (N=173) | |
| Mean ± SD | 26.7 ± 4.51 |
| Pre-operative Cardiac Diagnosis | |
| Idiopathic Dilated Cardiomyopathy | 76 (38.0) |
| Ischemic Cardiomyopathy | 70 (35.0) |
| Other | 54 (27.0) |
| LVAD Support at the time of transplant | 72 (36.0) |
| UNOS status at the time of transplant | |
| 1A | 110 (55.0) |
| 1B | 78 (39.0) |
| 2 | 12 ( 6.0) |
| CMV IgG Status [1] | |
| Positive | 112 (56.0) |
| Negative | 86 (43.0) |
| Donor,Recipient CMV IgG Status | |
| D+,R− | 53 (26.5) |
| D+,R+ | 64 (32.0) |
| D−,R+ | 47 (23.5) |
| D−,R− | 31 (15.5) |
| Use of Induction Therapy (N=194) | |
| Anti-IL-2R | 35 (18.0) |
| ATG | 49 (25.3) |
| Anti-IL-2R or ATG | 83 (42.8) |
| Use of Maintenance Therapy within 1st Month (N=176) | |
| Tacrolimus/Cyclosporine | 173 (98.3) |
| MMF | 173 (98.3) |
| Steroids | 172 (97.7) |
Three subjects have no donor CMV status summarized here: 1 reported as ‘Indeterminate’ and 2 reported as ‘Not Done’.
Two subjects have no recipient CMV status summarized here: 1 reported as ‘Not Done’ and 1 is missing.
Post-transplant immunosuppression was determined by local practice (Table 1): 25.3% of subjects received induction therapy with rabbit anti-thymocyte globulin (ATG) and 18% received anti-IL-2 receptor (anti-IL-2R) induction. Maintenance immunosuppression varied among centers but generally included tacrolimus, mycophenolate mofetil (MMF) or its equivalent and a variable course of corticosteroids. Immunosuppression at one site differed from the others: no high dose steroids were administered at transplant, no ATG and/or anti-IL-2R was used for induction, all recipients were treated with MMF for 3–6 weeks only and post-transplant corticosteroids were administered for 8–12 weeks only; tacrolimus was initiated at the time of transplantation and was used as the only maintenance therapy beyond 8–12 weeks.
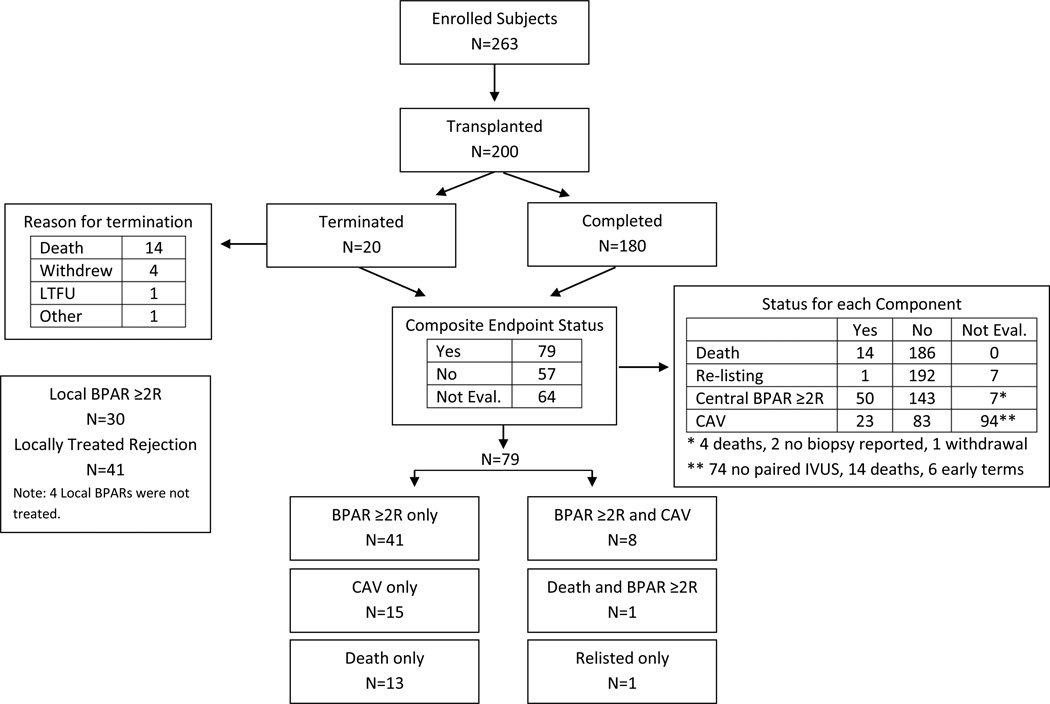
The 12-month outcomes of the 200 transplanted subjects included 14 deaths, 1 re-listing for transplantation, and 50 subjects with at least one episode of BPAR >2R (Figure 1).
Figure 1. Overview of study outcomes.
Consort diagram illustrating the outcomes of subjects throughout the course of the study including numbers and results of biopsies performed and numbers of subjects who reached the 12-month endpoint. 0f 200 transplanted subjects (and of 180 who completed the study), 64 were not evaluable for the primary composite endpoint because IVUS data were missing (not evaluable for the CAV component) and the subject did not have an episode of BPAR >2R. Subjects without IVUS data but who had an episode of BPAR component were considered evaluable because they met the BPAR component of the composite endpoint.
106 of the 200 transplanted subjects had evaluable paired IVUS studies (Figure 1, Table 2), a similar percentage as reported in published studies (52–54). Clinical characteristics of the CAV subset were similar to those not evaluable for CAV with the exception of a lower frequency of LVAD support, less ATG induction, and a higher prevalence of CMV-IgG positivity (not shown). Twenty-three of the 106 subjects (22%) met the CAV endpoint. 79 of 136 evaluable subjects reached the composite endpoint (Figure 1, Table 2).
Table 2.
Baseline Characteristics of Study Subjects Stratified by Primary Composite, Central BPAR, and CAV Endpoints
| Characteristic | Primary Composite Endpoint = Yes (N=79) |
Primary Composite Endpoint = No (N=57) |
p-val | Central BPAR ≥2R = Yes (N=50) |
Central BPAR ≥2R = No (N=143) |
p-val | CAV Endpoint = Yes (N=23) |
CAV Endpoint = No (N=83) |
p-val |
|---|---|---|---|---|---|---|---|---|---|
| Donor Characteristics | |||||||||
| Age | |||||||||
| Mean ± SD | 33.3 ± 11.53 | 28.8 ± 11.32 | 0.027 | 32.5 ± 11.56 | 30.5 ± 12.01 | 0.313 | 34.1 ± 10.96 | 29.6 ± 11.62 | 0.099 |
| Male Gender | 53 (67.1) | 39 (68.4) | 0.870 | 31 (62.0) | 102 (71.3) | 0.220 | 16 (69.6) | 54 (65.1) | 0.686 |
| Race | |||||||||
| White | 55 (69.6) | 39 (68.4) | 0.264 | 33 (66.0) | 99 (69.2) | 0.257 | 18 (78.3) | 54 (65.1) | 0.482 |
| Black or African American | 15 (19.0) | 7 (12.3) | 12 (24.0) | 19 (13.3) | 3 (13.0) | 15 (18.1) | |||
| Other | 1 ( 1.3) | 3 ( 5.3) | 1 ( 2.0) | 5 ( 3.5) | 0 | 3 ( 3.6) | |||
| Unknown or Not Reported | 8 (10.1) | 8 (14.0) | NA | 4 ( 8.0) | 20 (14.0) | NA | 2 ( 8.7) | 11 (13.3) | NA |
| Ethnicity | |||||||||
| Hispanic or Latino | 14 (17.7) | 12 (21.1) | 0.641 | 8 (16.0) | 25 (17.5) | 0.860 | 6 (26.1) | 16 (19.3) | 0.557 |
| Not Hispanic or Latino | 56 (70.9) | 39 (68.4) | 35 (70.0) | 101 (70.6) | 15 (65.2) | 60 (72.3) | |||
| Unknown or Not Reported | 9 (11.4) | 6 (10.5) | NA | 7 (14.0) | 17 (11.9) | NA | 2 ( 8.7) | 7 ( 8.4) | NA |
| Cause of Death | |||||||||
| Anoxia | 16 (20.3) | 7 (12.3) | 0.281 | 10 (20.0) | 20 (14.0) | 0.563 | 4 (17.4) | 15 (18.1) | 0.599 |
| Cerebrovascular/Stroke | 10 (12.7) | 4 ( 7.0) | 5 (10.0) | 21 (14.7) | 3 (13.0) | 7 ( 8.4) | |||
| Head Trauma | 35 (44.3) | 34 (59.6) | 23 (46.0) | 74 (51.7) | 9 (39.1) | 44 (53.0) | |||
| Other | 18 (22.8) | 12 (21.1) | 12 (24.0) | 28 (19.6) | 7 (30.4) | 17 (20.5) | |||
| CMV IgG Status [1] | |||||||||
| Positive | 50 (63.3) | 34 (59.6) | 0.532 | 30 (60.0) | 84 (58.7) | 0.799 | 16 (69.6) | 50 (60.2) | 0.414 |
| Negative | 27 (34.2) | 23 (40.4) | 19 (38.0) | 58 (40.6) | 7 (30.4) | 33 (39.8) | |||
| Recipient Characteristics | |||||||||
| Age (year) | |||||||||
| Mean ± SD | 53.9 ± 12.30 | 53.5 ± 11.68 | 0.838 | 53.5 ± 12.86 | 53.9 ± 11.92 | 0.838 | 52.0 ± 12.04 | 53.4 ± 12.31 | 0.622 |
| Male Gender | 68 (86.1) | 43 (75.4) | 0.114 | 43 (86.0) | 113 (79.0) | 0.281 | 22 (95.7) | 63 (75.9) | 0.039 |
| Race | |||||||||
| White | 58 (73.4) | 44 (77.2) | 0.234 | 37 (74.0) | 108 (75.5) | 0.624 | 19 (82.6) | 61 (73.5) | 0.371 |
| Black or African American | 13 (16.5) | 5 ( 8.8) | 10 (20.0) | 20 (14.0) | 1 ( 4.3) | 13 (15.7) | |||
| Other | 3 ( 3.8) | 5 ( 8.8) | 3 ( 6.0) | 6 ( 4.2) | 2 ( 8.7) | 6 ( 7.2) | |||
| Unknown or Not Reported | 5 ( 6.3) | 3 ( 5.3) | NA | 0 | 9 ( 6.3) | NA | 1 ( 4.3) | 3 ( 3.6) | NA |
| Ethnicity | |||||||||
| Hispanic or Latino | 2 ( 2.5) | 5 ( 8.8) | 0.238 | 2 ( 4.0) | 11 ( 7.7) | 0.736 | 1 ( 4.3) | 5 ( 6.0) | >0.999 |
| Not Hispanic or Latino | 68 (86.1) | 49 (86.0) | 41 (82.0) | 127 (88.8) | 21 (91.3) | 73 (88.0) | |||
| Unknown or Not Reported | 9 (11.4) | 3 ( 5.3) | NA | 7 (14.0) | 5 ( 3.5) | NA | 1 ( 4.3) | 5 ( 6.0) | NA |
| Weight (kg) | N=72 | N=55 | 0.010 | N=45 | N=136 | 0.029 | N=21 | N=79 | 0.088 |
| Mean ± SD | 85.0 ± 16.80 | 77.4 ± 15.94 | 86.2 ± 17.47 | 80.3 ± 14.94 | 85.9 ± 17.19 | 78.9 ± 16.59 | |||
| BMI (kg/m²) | N=63 | N=50 | 0.048 | N=38 | N=128 | 0.165 | N=20 | N=69 | 0.285 |
| Mean ± SD | 27.5 ± 4.35 | 25.8 ± 4.69 | 27.6 ± 4.56 | 26.4 ± 4.58 | 27.4 ± 4.62 | 26.1 ± 4.79 | |||
| Pre-operative Cardiac Diagnosis | |||||||||
| Idiopathic Dilated Cardiomyopathy | 30 (38.0) | 23 (40.4) | 0.785 | 22 (44.0) | 54 (37.8) | 0.420 | 7 (30.4) | 36 (43.4) | 0.380 |
| Ischemic Cardiomyopathy | 28 (35.4) | 17 (29.8) | 19 (38.0) | 50 (35.0) | 10 (43.5) | 24 (28.9) | |||
| Other | 21 (26.6) | 17 (29.8) | 9 (18.0) | 39 (27.3) | 6 (26.1) | 23 (27.7) | |||
| LVAD Support at the time of transplant | 30 (38.0) | 13 (22.8) | 0.061 | 19 (38.0) | 50 (35.0) | 0.700 | 7 (30.4) | 22 (26.5) | 0.708 |
| UNOS status at the time of transplant | |||||||||
| 1A | 48 (60.8) | 28 (49.1) | 0.006 | 32 (64.0) | 72 (50.3) | 0.151 | 10 (43.5) | 47 (56.6) | 0.056 |
| 1B | 30 (38.0) | 20 (35.1) | 17 (34.0) | 60 (42.0) | 13 (56.5) | 27 (32.5) | |||
| 2 | 1 ( 1.3) | 9 (15.8) | 1 ( 2.0) | 11 ( 7.7) | 0 | 9 (10.8) | |||
| CMV IgG Status [1] | |||||||||
| Positive | 37 (46.8) | 43 (75.4) | <0.001 | 24 (48.0) | 84 (58.7) | 0.172 | 10 (43.5) | 57 (68.7) | 0.027 |
| Negative | 42 (53.2) | 14 (24.6) | 26 (52.0) | 58 (40.6) | 13 (56.5) | 26 (31.3) | |||
| Donor,Recipient CMV IgG Status | |||||||||
| D+,R− | 26 (32.9) | 9 (15.8) | 15 (30.0) | 38 (26.6) | 8 (34.8) | 16 (19.3) | |||
| D+,R+ | 24 (30.4) | 25 (43.9) | 0.015 | 15 (30.0) | 46 (32.2) | 0.566 | 8 (34.8) | 34 (41.0) | 0.112 |
| D−,R+ | 13 (16.5) | 18 (31.6) | 9 (18.0) | 37 (25.9) | 2 ( 8.7) | 23 (27.7) | |||
| D−,R− | 14 (17.7) | 5 ( 8.8) | 10 (20.0) | 20 (14.0) | 5 (21.7) | 10 (12.0) | |||
| Use of Induction Therapy | N=73 | N=57 | N=49 | N=139 | N=22 | N=83 | |||
| Anti-IL-2R | 10 (13.7) | 18 (31.6) | 0.014 | 6 (12.2) | 28 (20.1) | 0.217 | 5 (22.7) | 19 (22.9) | 0.987 |
| ATG | 11 (15.1) | 15 (26.3) | 0.112 | 5 (10.2) | 42 (30.2) | 0.005 | 1 ( 4.5) | 18 (21.7) | 0.070 |
| Anti-IL-2R or ATG | 20 (27.4) | 33 (57.9) | <0.001 | 11 (22.4) | 70 (50.4) | <0.001 | 6 (27.3) | 37 (44.6) | 0.142 |
Counts of ‘Indeterminate’ and ‘Not Done’ results are not presented here.
Clinical characteristics and outcomes
Clinical characteristics associated with the primary composite endpoint (Table 2) included older donor age (33.3±11.3 vs. 28.8±11.32 years, p=0.03), higher recipient weight and body mass index (p <0.05 for each), recipient waiting list status (p=0.006), recipient CMV-negative status (regardless of donor status, p<0.001, Figure S2), and absence of induction therapy with either anti-IL-2R or ATG (use of either was associated with a lower rate of reaching the composite endpoint, p<0.001, Table 2). Pre-transplant LVAD support trended toward significance (p=0.06). A higher frequency of recipient CMV-negative but donor CMV-positive (D+R−) subjects met the composite endpoint compared to CMV+ recipients regardless of donor status (74% vs. 46%, p=0.005, univariate analyses, Figure S2). Post-transplant CMV infection was diagnosed in 12 subjects. We did not observe significant correlations between CMV infection and BPAR or CAV (only 7/12 had IVUS data evaluable for CAV, not shown).
Regarding the individual components of the composite, recipient weight correlated directly with developing >1 episode of BPAR, while induction therapy was associated with a lower incidence of BPAR (Table 2). CAV occurred more commonly in males (p=0.039) and in CMV− recipients (p=0.03). CAV trended higher in CMV− recipients who had CMV+ donors as compared to CMV+ recipients regardless of donor status (33% vs. 15%, p=0.073, Figure S3). We also observed a trend toward a lower incidence of CAV in subjects given ATG (p=0.07, Table 2).
Serum antibodies and outcomes
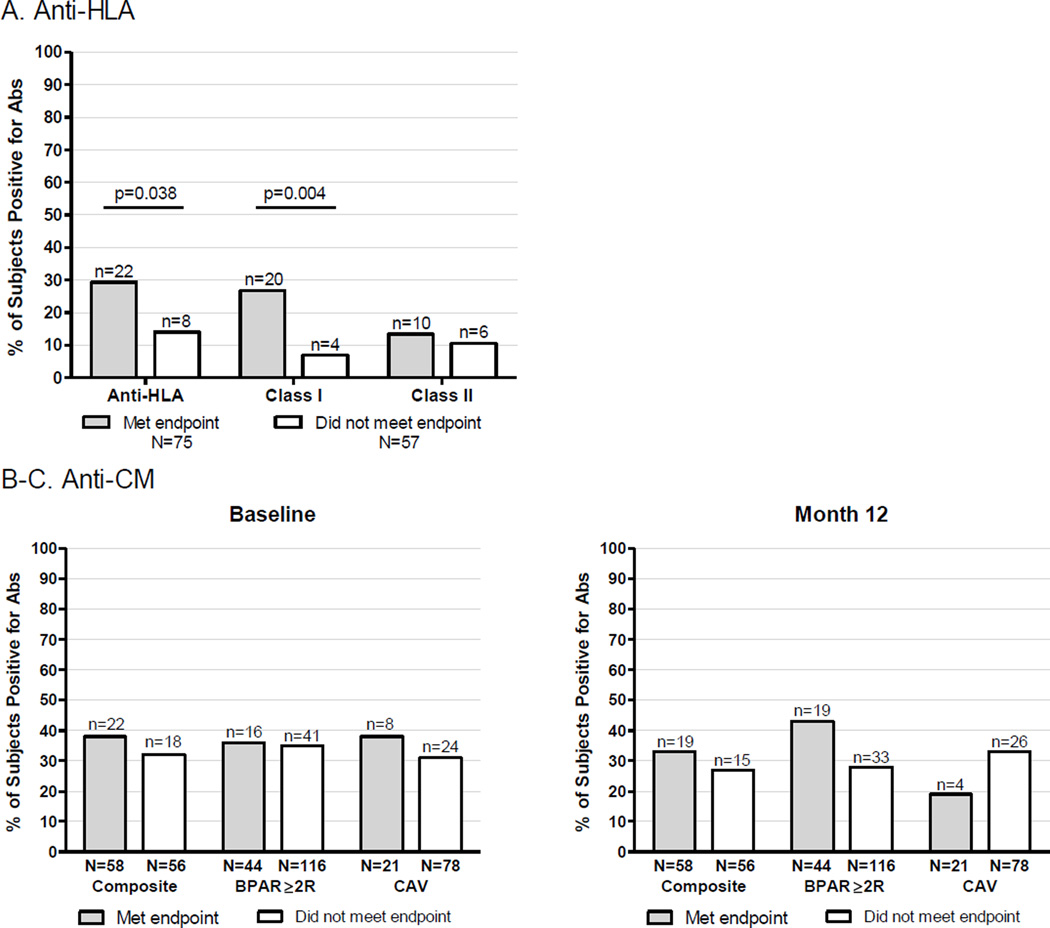
We detected serum anti-HLA antibodies in 24% (46/195) of subjects either pre- or post-transplantation. 12% (n=24) were reactive to class I HLA alone, 6% (n=11) to class II HLA alone and 6% (n=11) to both class I and II. There were 132 subjects evaluable for the primary composite endpoint and who had serum samples available for analysis (Figure 2A). The prevalence of anti-HLA antibodies was greater among those who met the endpoint (22/75, 29%) than among those who did not (8/57, 14%, p=0.04). Anti-class I antibodies were present in 20/75 (27%) subjects who met the endpoint vs. 4/57 (7%) who did not (p<0.01). There were no differences in anti-class II antibodies between subjects who met the endpoint (10/75, 13%) and those who did not (6/57, 11%, p=ns).
Figure 2. Relationships between serum anti-HLA or anti-CM antibodies and study outcomes.
A. Percentages of study subjects with any serum anti-HLA antibodies (left), antibodies reactive to class I HLA (middle) and antibodies reactive to class II HLA (right), stratified by meeting (gray) or not meeting (white) the composite endpoint. B-C. Percentages of study subjects with serum anti-CM antibodies at baseline (B) or at 12-months (C) who met (gray) or did not meet (white) the composite endpoint (left), BPAR endpoint (middle), CAV endpoint (right). Anti-VM antibodies were also tested baseline and 12-months, and no relationships were observed with any of the endpoints at either time point (not shown).
Of 193 evaluable subjects with donor typing available, serum from 21 subjects contained DSA. In 14 instances DSA was present in the pre-transplant sample. In 6 subjects DSA developed de novo post-transplant. In 1 subject DSA was detected post-transplant but the pre-transplant sample was not adequate (we could not determine the timing of DSA development). Of 130 subjects evaluable for the primary composite endpoint with available donor/recipient HLA typing data, DSA was present in 15: 10/15 (67%) subjects with DSA met the endpoint vs. 64/115 (56%) subjects without DSA (p=ns). Within the 104 subjects evaluable for the CAV endpoint, 2/11 with DSA met the endpoint compared with 21/93 without DSA (p=ns).
In an effort to extend/validate previous studies suggesting that autoantibodies reactive to VM or CM correlate with worse heart transplant outcomes (34–39), we quantified serum anti-VM and anti-CM auto-antibodies pre-transplant and at 12-months post-transplant and correlated the results with the 1-year outcomes. Neither positive serum anti-CM antibodies (Figure 2B) nor positive serum anti-VM antibodies (data not shown) were associated meeting the composite endpoint. We did not observe associations between anti-CM autoantibody (Figure 2B) or anti-VM (not shown) and the incidence of BPAR or CAV.
Cellular alloimmunity and outcomes
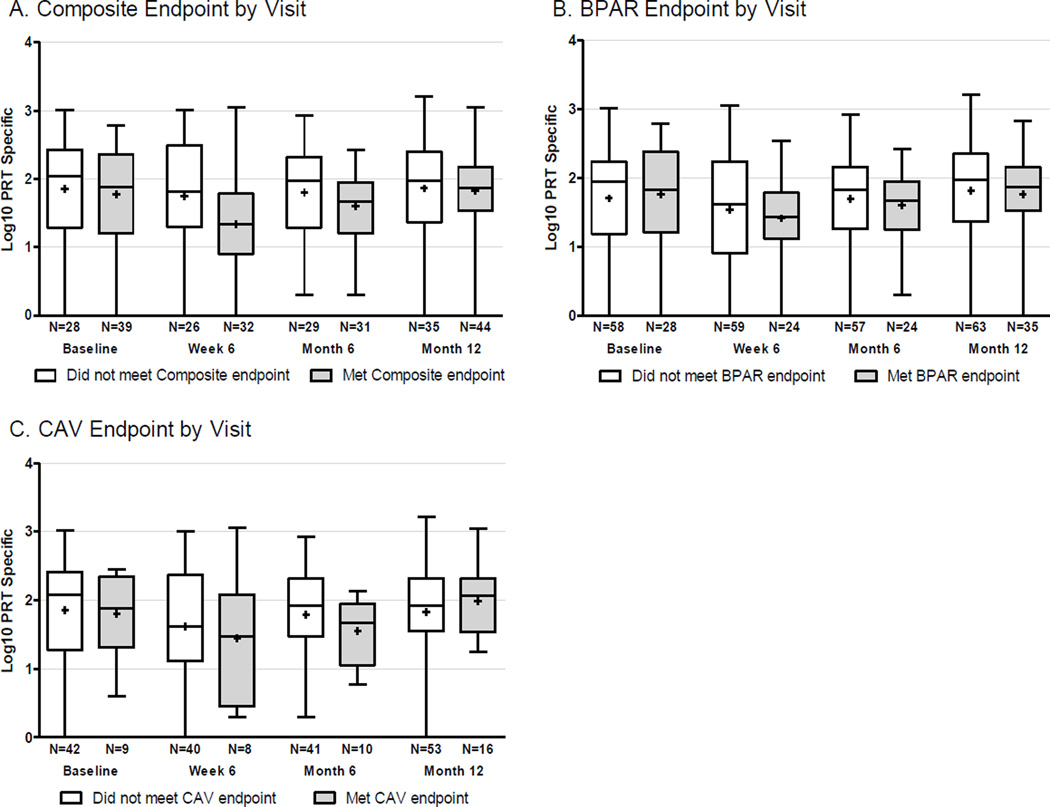
Based on previous work showing frequencies of alloreactive IFNγ-producing PBMCs correlated with worse outcomes in kidney transplant recipients (55, 56), we determined the frequency of primed/memory cellular alloimmunity pre-transplant using an IFNγ-ELISPOT assay. Low rates of donor cell collection prevented us from delineating frequencies of donor-reactive cellular immunity in the recipients. As an alternative, we quantified allo-reactivity by stimulating PBMC with a panel of allogeneic stimulator cells as described in panel of reactive T cells (PRT) assay (51, 55, 56). Interpretable assays were available from 130 subjects. We did not observe significant associations between the strength of the pre-transplant, or the post-transplant, PRT and either the composite endpoint, BPAR >2R, or CAV (Figure 3). This was true regardless of whether the PRT was analyzed as a continuous variable or as a dichotomous variable based on a pre-defined threshold derived from kidney transplant recipients (51).
Figure 3. Panel of reactive T cell (PRT) and study outcomes.
Frequencies of alloreactive IFNγ-producing PBMCs at baseline, 6-weeks, 6-months and 12-months post-transplant who met (grey) or did not meet (white) the composite endpoint (A), the BPAR endpoint (B) or CAV endpoint (C). The values (n) below each bar represent the number of subjects with available ELISPOT results who were evaluable for each endpoint. There were no statistically significant differences among groups.
Peripheral blood and tissue gene expression profiles and outcomes
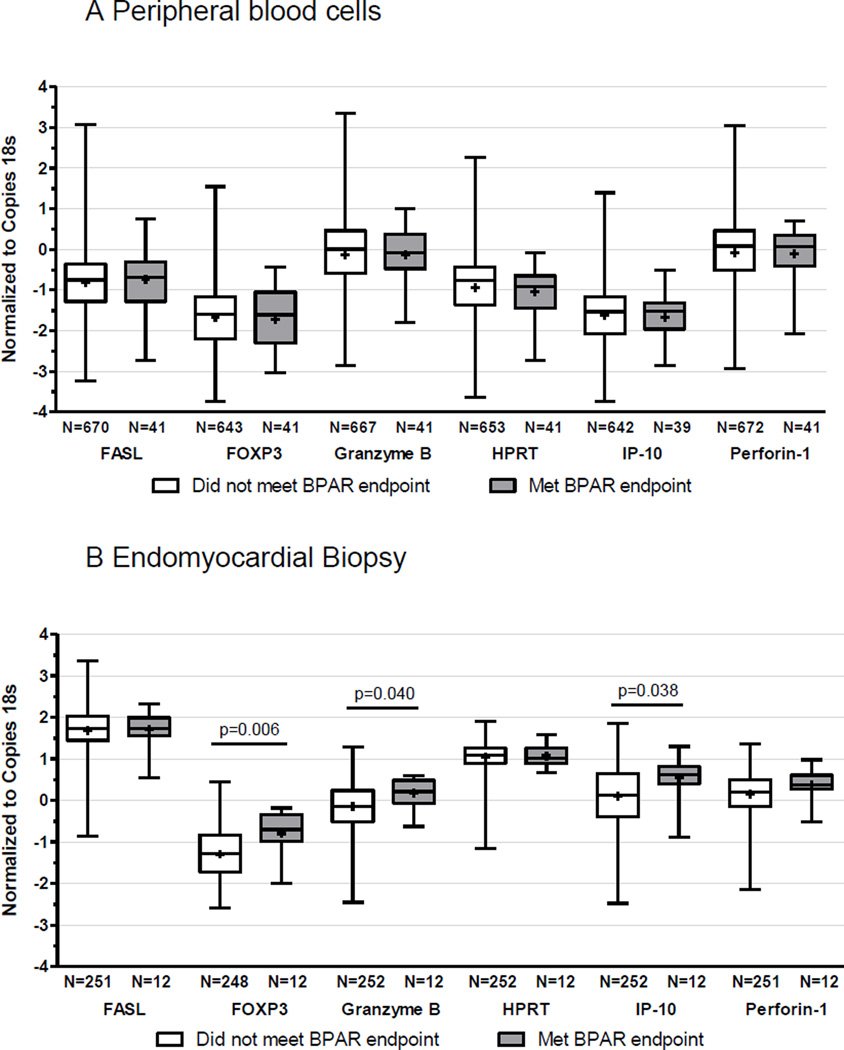
Based on previous publications indicating that various effector T cell gene expression patterns detected in peripheral blood cells correlate with BPAR (57, 58) we serially quantified peripheral blood expression of 6 candidate genes, FasL, Foxp3, GZMB, HPRT, CXCL10, and PRF1 in our study cohort, and correlated the results with the rejection outcomes. We observed no significant correlations between any of the mRNAs and BPAR ≥2R at the time of rejection (Figure 4A). Nor did we observe any correlations between any of the peripheral blood cell mRNA levels and CAV (data not shown).
Figure 4. Gene expression profiling and study outcomes.
A. Absolute copy numbers for each of the listed genes normalized to the copy number of 18S–rRNA in peripheral blood cell samples obtained up to the first episode of BPAR in each individual, stratified by meeting (gray) or not meeting (white) the BPAR endpoint. The values (n) below each bar represent the number of evaluable subjects with available PCR results. B. Absolute copy numbers for each of the listed genes normalized to the copy number of 18S–rRNA in endomyocardial tissue samples obtained at the time of protocol or for-cause biopsy <2 weeks prior to obtaining a biopsy sample that were read by the core pathology laboratory as meeting the BPAR endpoint (gray) or not (white). The values (n) below each bar represent the number of evaluable subjects with available PCR results.
When we examined expression patterns of the same genes within biopsy tissue with and without BPAR, we did observe significantly higher levels of granzyme B, Foxp3 and CXCL10/IP-10 mRNA in association with BPAR ≥2R (Figure 4B). The expression levels of individual genes in the peripheral blood did not correlate with their expression levels in cardiac tissue (all correlation values were r<0.2).
We extended the analysis to include a larger number of potential biomarker genes using Nanostring®, quantifying >500 immune-related RNAs from serially collected peripheral blood samples using a subset of 9 subjects without BPAR or CAV and 9 subjects with at least one episode of BPAR. Again, the analyses did not show any significant relationships (data not shown). We did not specifically evaluate the association of gene used in the AlloMap® assay with BPAR, but 4/11 genes in AlloMap® (PDCD1, ITGAM, ITGA4, IL1R2) were present in the CTOT-05 nanostring panel. We performed univariate and multivariate logistic regression using these 4 markers to test for associations with central BPAR and locally treated rejection; none were significant univariately or in combination with others.
Plasma levels of angiogenesis-related proteins and CAV
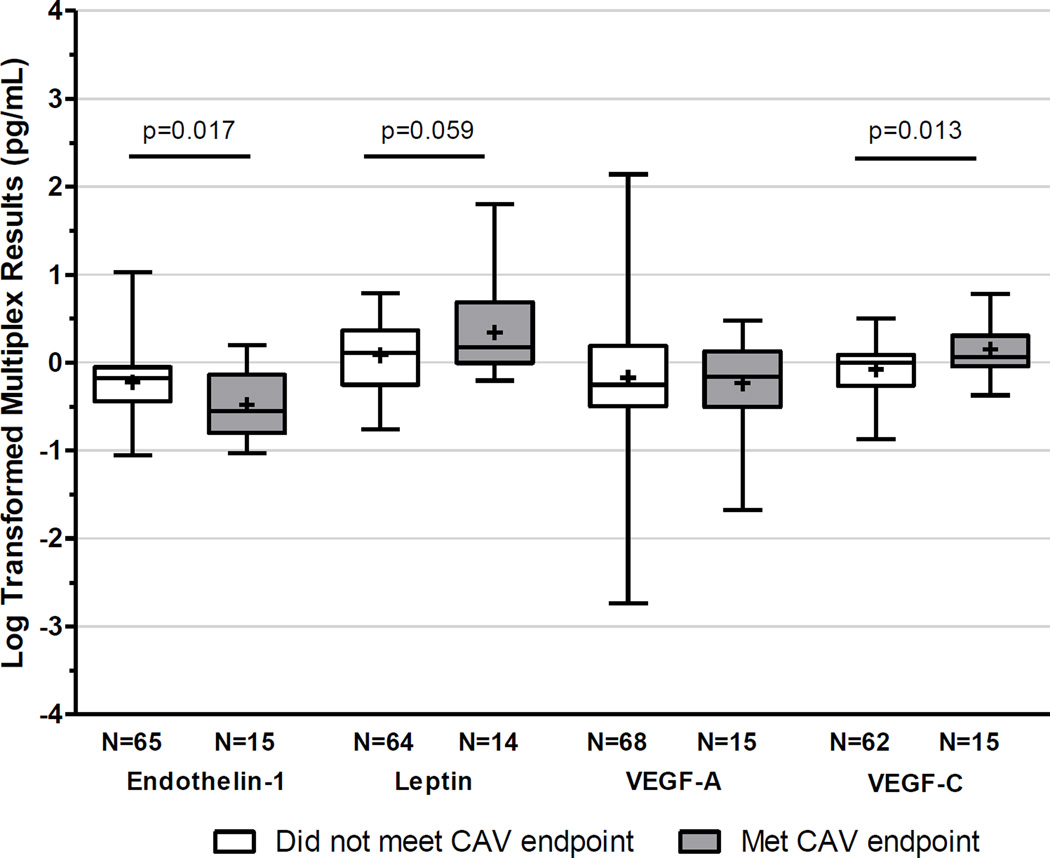
We performed a Luminex screen for 17 angiogenesis/vascular injury-related proteins (Table 3) using plasma from a subset of subjects within the IVUS cohort (n=46 with paired samples). While none of the markers individually correlated with the development of CAV, multivariate analysis showed increases in VEGF-C (OR: 2.7; 95% CI 1.15–6.23) and FGF1 (OR: 14.1; 95% CI 1.06–188) combined with decreases in ET-1 (OR: 0.2; 95% CI 0.06–0.71) from pre-transplant to 12-months post-transplant were associated with CAV (Table 3). We subsequently performed ELISAs for ET-1, Leptin, VEGF-C and VEGF-A on all plasma samples obtained at study entry and 12-mo post-transplant from the subjects with paired IVUS evaluations (Figure 5, Table 4). The baseline plasma protein concentrations did not predict CAV, but increases in VEGF-C (p=0.013) and decreases in serum ET-1 (p=0.017) correlated with the development of CAV. Multivariable regression analyses (Table 4) that included clinical characteristics identified in Table 2 showed that the changes in VEGF-C (OR 20; 95% CI 1.9–218) and ET-1 (OR 0.14; 95% CI 0.02–0.97) were associated with CAV independent of ATG induction, CMV status or male sex. Changes in ET-1 or VEGF-C did not correlate with the composite endpoint.
Table 3.
Change in plasma concentrations of angiogenesis-related proteins (Luminex) from pre-transplant to 1 year post-transplant is associated with the development of CAV by IVUS
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | n | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Change in Angiopoietin-2 | 46 | 1.50 | 0.22–10.49 | 0.680 | |||
| Change in BMP-9 | 46 | 1.11 | 0.21–5.90 | 0.906 | |||
| Change in EGF | 46 | 2.01 | 0.51–7.98 | 0.319 | |||
| Change in Endoglin | 46 | 0.86 | 0.36–2.08 | 0.738 | |||
| Change in Endothelin-1 | 46 | 0.50 | 0.21–1.20 | 0.119 | 0.20 | 0.06–0.71 | 0.012 |
| Change in FGF-1 | 46 | 3.45 | 0.47–25.17 | 0.221 | 14.11 | 1.06–188.22 | 0.045 |
| Change in FGF-2 | 46 | 3.74 | 0.08–168.41 | 0.497 | |||
| Change in Follistatin | 46 | 0.80 | 0.25–2.56 | 0.704 | |||
| Change in G-CSF | 46 | 0.78 | 0.30–2.01 | 0.606 | |||
| Change in HB-EGF | 46 | 5.25 | 0.20–138.31 | 0.320 | |||
| Change in HGF | 46 | 0.45 | 0.18–1.17 | 0.103 | |||
| Change in IL-8 | 46 | 1.10 | 0.53–2.26 | 0.803 | |||
| Change in Leptin | 46 | 1.89 | 0.37–9.66 | 0.442 | |||
| Change in PLGF | 46 | 1.06 | 0.55–2.05 | 0.860 | |||
| Change in VEGF-A | 46 | 1.20 | 0.61–2.37 | 0.599 | |||
| Change in VEGF-C | 46 | 1.69 | 0.86–3.33 | 0.128 | 2.68 | 1.15–6.23 | 0.022 |
| Change in VEGF-D | 46 | 1.33 | 0.42–4.22 | 0.632 | |||
N= 46 subjects. Subjects were randomly selected from the CAV cohort who met or did not meet the endpoint (2:1 ratio).
Figure 5. Plasma angiogenesis-related proteins and CAV.
Change in plasma concentration by ELISA for each of the listed proteins between the pre-transplant and 1 year post-transplant visit stratified by meeting (gray) or not meeting (white) the CAV endpoint. The values (n) below each bar represent the number of IVUS evaluated subjects with available paired ELISA results.
Table 4.
Change in plasma concentrations of angiogenesis-related proteins (ELISA) from pre-transplant to 1 year post-transplant is associated with the development of CAV by IVUS
| Univariate |
Multivariate (n=74) |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | n | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Male | 106 | 6.98 | 0.88–55.13 | 0.065 | |||
| CMV+ Recipient | 106 | 0.35 | 0.14–0.90 | 0.030 | 0.35 | 0.09–1.28 | 0.112 |
| ATG Induction | 105 | 0.17 | 0.02–1.37 | 0.096 | |||
| Change in Endothelin-1 | 80 | 0.12 | 0.02–0.69 | 0.017 | 0.14 | 0.02–0.97 | 0.047 |
| Change in Leptin | 78 | 3.71 | 0.95–14.49 | 0.059 | |||
| Change in VEGF-A | 83 | 0.90 | 0.43–1.86 | 0.770 | |||
| Change in VEGF-C | 77 | 15.98 | 1.79–142.49 | 0.013 | 20.11 | 1.85–218.04 | 0.014 |
N=106 subjects with IVUS evaluations
Discussion
CTOT-05 was designed as a multicenter, observational study to assess relationships between markers and endpoints in a group of first heart transplant recipients treated with heterogeneous immunosuppression and CMV prophylaxis that reflects current standard of care in the US. In contrast to findings from previous, predominantly small, single-center biomarker reports of heart transplant recipients (4–20), the results of CTOT-05 did not show significant associations between the majority of the tested biomarkers and the composite endpoint or BPAR. Our findings suggest that the majority of the tested biomarkers are unlikely to be clinically useful surrogates for outcomes.
One weakness of the study design derives from heterogeneity in practice among study centers, including suboptimal standardization of clinically relevant endpoints. With regard to BPAR, significant variability of endomyocardial biopsy interpretation among expert pathologists reading the same biopsy slides has been previously documented (59, 60). In CTOT-05, treatment decisions were based on local rather than core lab biopsy interpretation, and thresholds for initiating treatment likely differed among sites. Core lab biopsy diagnoses derived from analyses of sections from the same tissue block as those used for the local reading, but for some centers, unique sections were sent to the core lab, adding to variability. These differences likely contributed to the observed discordance between central reads of BPAR>2R and local decisions to treat for rejection (Figure S4), which could have confounded detection of associations between biomarkers and the composite endpoint and/or the BPAR component. In support of this concept a previously published report (59) indicated that a diagnosis of BPAR ≥2R made in isolation (without considering clinical context) was deemed insufficient for making clinical decisions or for use as a research criterion. We speculate that previously reported biomarker analyses from single center heart transplant studies provided more consistent correlations with BPAR because the grading of BPAR and the decision to treat are more uniform at a single site.
Regarding the IVUS component of the endpoint, while changes in IVUS measurements over 1–2 years post-transplant have been shown to be associated with graft survival (44, 61–65), limitations of using this technique for biomarker analyses also exist. The percentage of subjects with interpretable IVUS results in our study (53%) was similar to that previously reported from multicenter studies (53). Nonetheless, with 106 interpretable IVUS pairings and a lower than anticipated rate of developing CAV (23/106, ~20%), absence of detected associations between CAV and the tested biomarkers could have been a result of a type 2 error. In addition, the IVUS-defined endpoint in CTOT-05 was a measured increase in maximal intimal thickness of >0.5 mm in a matched coronary segment over 1 year. In the ensuing years since the initiation of CTOT-05 the heart transplant research community has adopted volumetric analyses as more sensitive and reliable measures of CAV than measurements of intimal thickness (66). Combined with other documented limitations of IVUS (67) and known coefficients of variance of most biomarkers of ~30% (50, 68), our findings suggest that future multicenter studies of biomarkers in heart transplantation may need to include several hundred evaluable subjects studied with volumetric-based IVUS analyses to have sufficient power to identify meaningful relationships with CAV.
One CTOT-05 study site employed a nontraditional immunosuppression protocol (no induction and tacrolimus only) that could have influenced detectable relationships between biomarkers and outcomes in the entire cohort. However, when we re-analyzed the data excluding subjects from this site we observed similar relationships compared to those observed in the entire cohort (not shown).
One perceived strength of CTOT-05 is that it was intentionally designed to identify biomarkers of heart transplant outcomes in the context of current clinical practice, which involves heterogeneous immunosuppression and CMV prophylaxis/therapy protocols. We acknowledge the possibility that the tested biomarkers may behave differently if immunosuppression/CMV therapy was identical across sites. Despite these acknowledged limitations we did observe that plasma levels of peripheral blood proteins associated with vascular injury and remodeling are promising biomarkers for development of CAV. Increases in the plasma VEGF-C together with decreases in ET1 (plasma ELISAs) over the first post-transplant year were strongly associated with developing CAV [AUC=0.794 from single model; 0.750 from bootstrapped model]. VEGF-C is a well-established growth factor for lymphatic endothelial cells, and overexpression has been previously reported to contribute to immune-mediated chronic allograft injury (69, 70). The associated decreases in ET1 were not predicted from the known angiogenic mechanisms of action of this protein (71) and mechanistic links with VEGF-C remain speculative. Several studies have identified additional vascular growth factors including VEGF-A in blood (33, 72) and tissue (20, 69, 73, 74) as biomarkers of established CAV at later times post-transplantation. Most of these studies, including a 2013 publication (33) showed relationships between the markers and established angiographic CAV >5 years after transplantation. Our findings extend this and other previous work (10) by showing that select angiogenesis markers in the peripheral blood have the potential to inform risk of incipient CAV during the initial post-transplant year. Longer term follow-up will be required to directly assess relationships of angiogenesis markers obtained within the first year to the incidence of major adverse cardiac events detected at >2 years and to graft survival. Toward this end, an analysis of relationships between biomarkers obtained within the initial year post-transplant and 4-year outcomes of the CTOT-05 cohort is ongoing (CTOT-18, www.ctot.org).
Previous studies by our group among others (50, 51, 75–77) indicate that many of the tested biomarkers can provide diagnostic/prognostic information in kidney transplant recipients, raising the additional possibility that absence of correlations in the CTOT-05 cohort reflects organ specific differences. On the other hand, our observation that 3 genes found to be informative in acute kidney injury (Foxp3, GZMB and CXCL10 (78, 79) were significantly upregulated in heart graft tissue with histological evidence of acute cellular rejection (and the absence of a correlation between expression levels in the blood and the tissue, not shown) suggests that local events within the graft may result in the similar alloactivation of a subset(s) of T cells but that they cannot be detected in peripheral blood. The absence of detectable correlations between peripheral blood gene expression profiles and histological BPAR and/or IVUS-defined CAV in CTOT-05 contrasts with previous studies that reported AlloMap® is a useful biomarker in heart transplantation (3). We speculate that one key reason for what would appear as disparate conclusions is that the AlloMap® was shown to limit the requirement for endomyocardial biopsies (3) as opposed to being a diagnostic marker for rejection and/or CAV. We did not include the AlloMap® or all of its gene targets in the CTOT-05 analysis and are thus unable comment on AlloMap®’s diagnostic utility. We observed an association between serum anti-HLA antibodies and rejection/CAV, which together with results from other studies (80–82) supports the conclusion that anti-HLA antibodies are pathogenic in human heart transplant recipients. While the CTOT-05 analyses did not demonstrate a statistically significant association between DSA and outcomes, few evaluable subjects (n=15) developed DSA over this 12-month study, further supporting the need for evaluating larger cohorts followed for longer time periods.
The CTOT-05 results validate and extend previously identified clinical characteristics associated with an elevated risk of graft loss/death, BPAR or CAV (1). These factors include older donor age, recipient BMI, and worse pre-transplant clinical status as indicated by more urgent UNOS status and LVAD support (Table 1). Recipient CMV-negative serum status was also strongly associated with reaching these endpoints, consistent with previous studies (83–85). While the findings from our study suggest that subjects given ATG induction may be protected from developing CAV, CTOT-05 was not designed to prospectively test this. We therefore caution the heart transplant community from reaching any conclusions about the efficacy of ATG induction based on our results.
In summary, the CTOT-05 findings indicate that reliable biomarkers for heart transplant outcomes remain elusive. They also highlight the limitations of BPAR and traditionally analyzed IVUS measurements as endpoints in multicenter, observational, biomarker studies. Suggestive evidence that plasma levels of VEGF-C and ET-1 along with serum alloantibodies identify heart transplant recipients at elevated risk for allograft injury require further validation. The CTOT-05 results provide useful lessons for improving future design of biomarker validation trials and biomarker-guided interventional trials in heart transplantation.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under U01AI063594 awarded to P. Heeger and AI063623 awarded to M Sayegh. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The CTOT05 consortium members thank the following personnel for their support of the work: Beth Israel Deaconess Medical Center, Boston MA: Lingzhi Ma; Boston Children’s Hospital, Boston MA, USA: Katiana Calzadilla, Kayla McCleod, Sydney Keen; Brigham and Women’s Hospital, Boston, MA, USA: Usaila Ahmed, Nader Nafafian, Suzanne Kelly, and Brian Smith; Cleveland Clinic, Cleveland OH, USA: Robert Fairchild, Teresa Fonk, Barbara Gus, Karen Keslar, Bill Magyar, Emilio Poggio; Icahn School of Medicine at Mount Sinai, NY, NY, USA: Garrett Bell-Gresham, Brandy Haydel, Sherif Mikhail, Katya Ostrow, Riddikha Pandya, Denise Peace, Yasir Qureshi, Jennifer Smar, Paulina Trczinka, Tina Yao, Praeophayom Wauhop, Rosie Wickham; Loyola University Medical Center, Maywood, IL, USA: Carol Kartje; Medical City Dallas Hospital, Dallas TX, USA: Tina Worley; Intermountain Medical Center, Murray UT, USA: Kim Brunisholz and Joe Tuinei; Massachusetts General Hospital, Boston, MA, USA: Sandra L. Debronkart, Julie Finn and Jodi Titilah; Newark Beth Israel Medical Center, Newark NJ, USA: Jeanne Prevost-Fernandez, Bette Graves, Victor Costa; Northwestern University, Chicago IL, USA: Katy Prendergast and Shanna Davis; Rho, Chapel Hill, NC, USA: Danielle Boulet, Hyunsook Chin, Amy Tsay; University of California at San Francisco, San Francisco CA, USA: Erin Kobashigawa and Diana Hamilton; University of Manitoba, Winnipeg, Manitoba, Canada: Iga Dembinski and Peter Nickerson; University of Maryland, Baltimore MD,USA: Erika Feller MD,Linda Roma and Cindi Young; University of Utah Health Sciences Center, Salt Lake City UT, USA: Kimberley Davenport, Melissa Whipple, Jeff Gibbs.
Abbreviations
- Anti-IL-2R
anti-IL-2 receptor monoclonal antibody
- AR
acute rejection
- ATG
anti-thymocyte globulin
- BPAR
biopsy proven acute rejection
- CAV
coronary artery vasculopathy
- CM
cardiac myosin
- CMV
cytomegalovirus
- CTOT
Clinical Trials in Organ Transplantation
- DSA
donor specific antibody
- ET-1
endothelin-1
- FGF
fibroblast growth factor
- HLA
Human Leukocyte Antigen
- ISHLT
International Society of Heart and Lung Transplantation
- IVUS
intravascular ultrasound
- IFNγ
interferon gamma
- LVAD
left ventricular assist device
- MIT
maximal intimal thickness
- MMF
mycophenolate mofetil
- OR
odds ratio
- QA
quality assurance
- PBMC
peripheral blood mononuclear cells
- PRT
Panel of Reactive T cells
- VEGF
vascular endothelial growth factor
- VM
vimentin
*CTOT05 consortium, Site Principal Investigators
David A. Baran, Newark Beth Israel Medical Center, Newark NJ; William Cotts, Northwestern University, Chicago IL; Teresa De Marco, University of California at San Francisco, San Francisco CA; Michael Givertz, Brigham and Women’s Hospital, Boston, MA: Alain Heroux, Loyola University Medical Center, Maywood, IL; Judson Hunt, Medical City Dallas Hospital, Dallas TX; , A.G. Kfoury, Intermountain Medical Center, Murray UT; Joren Madsen, Massachusetts General Hospital, Boston, MA; Richard N. Pierson III, MD, University of Maryland, Baltimore MD; Sean Pinney, Icahn School of Medicine at Mount Sinai, NY, NY; Randall C. Starling, Cleveland Clinic, Cleveland OH; Josef Stehlik, Craig H Selzman, Edward M Gilbert, University of Utah Health Sciences Center, Salt Lake City UT
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
Supplemental Materials and Methods
Table S1. Eligibility Criteria
Figure S1. Tacrolimus Trough Levels by Visit. Box plots of the tacrolimus trough levels (mg/mL) at each of the five visits at which immunosuppressive trough levels were measured. The value of N below each box indicates the number of subjects who had a trough level reported at that time point. An ad hoc analysis looking at the relationship between ELISPOTs and trough levels showed there was no correlation between the post-transplant ELISPOTs and tacrolimus trough levels among the 271 observations that matched up in terms of time post-transplant (data not shown).
Figure S2. Donor, Recipient CMV Status at Transplant by Composite Endpoint. Numbers and percentages of subjects who met (gray) and did not meet (white) the composite endpoint when classified by donor, recipient CMV infection status.
Figure S3. Donor, Recipient CMV Status at Transplant by CAV Endpoint. Numbers and percentages of subjects who met (gray) and did not meet (white) the CAV endpoint when classified by donor, recipient CMV infection status.
Figure S4. Concordance of pathology scoring. Percent concordance (gray) and discordance (white) of the local pathology reads when compared to the central pathology reads using ISHLT acute cellular rejection scoring criteria.
References
- 1.Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33(10):996–1008. doi: 10.1016/j.healun.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report--2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951–964. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362(20):1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Stawinski GV, Cook DJ, Chui J, Gupta S, Navia JL, Hoercher K, et al. A comparative analysis between survivors and nonsurvivors with antibody mediated cardiac allograft rejection. The Journal of surgical research. 2007;142(2):233–238. doi: 10.1016/j.jss.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Yamani MH, Taylor DO, Haire C, Smedira N, Starling RC. Post-transplant ischemic injury is associated with up-regulated AlloMap gene expression. Clinical transplantation. 2007;21(4):523–525. doi: 10.1111/j.1399-0012.2007.00681.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamani MH, Taylor DO, Rodriguez ER, Cook DJ, Zhou L, Smedira N, et al. Transplant vasculopathy is associated with increased AlloMap gene expression score. J Heart Lung Transplant. 2007;26(4):403–406. doi: 10.1016/j.healun.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Yamani MH, Cook DJ, Rodriguez ER, Thomas DM, Gupta S, Alster J, et al. Increased expression of angiotensin II type 1 receptor (AGTR1) in heart transplant recipients with recurrent rejection. J Heart Lung Transplant. 2006;25(11):1283–1289. doi: 10.1016/j.healun.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Yousufuddin M, Yamani MH. The renin-angiotensin hypothesis for the pathogenesis of cardiac allograft vasculopathy. International journal of cardiology. 2004;95(2–3):123–127. doi: 10.1016/j.ijcard.2003.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Fahmy NM, Yamani MH, Starling RC, Ratliff NB, Young JB, McCarthy PM, et al. Chemokine and receptor-gene expression during early and late acute rejection episodes in human cardiac allografts. Transplantation. 2003;75(12):2044–2047. doi: 10.1097/01.TP.0000069601.73079.94. [DOI] [PubMed] [Google Scholar]
- 10.Ferri C, Properzi G, Tomassoni G, Santucci A, Desideri G, Giuliani AE, et al. Patterns of myocardial endothelin-1 expression and outcome after cardiac transplantation. Circulation. 2002;105(15):1768–1771. doi: 10.1161/01.cir.0000015606.69079.27. [DOI] [PubMed] [Google Scholar]
- 11.Yamani MH, Masri CS, Ratliff NB, Bond M, Starling RC, Tuzcu EM, et al. The role of vitronectin receptor (alphavbeta3) and tissue factor in the pathogenesis of transplant coronary vasculopathy. Journal of the American College of Cardiology. 2002;39(5):804–810. doi: 10.1016/s0735-1097(01)01823-x. [DOI] [PubMed] [Google Scholar]
- 12.Yen MH, Pilkington G, Starling RC, Ratliff NB, McCarthy PM, Young JB, et al. Increased tissue factor expression predicts development of cardiac allograft vasculopathy. Circulation. 2002;106(11):1379–1383. doi: 10.1161/01.cir.0000028588.73765.b4. [DOI] [PubMed] [Google Scholar]
- 13.Azzawi M, Hasleton PS, Turner DM, Yonan N, Deiraniya AK, Sinnott PJ, et al. Tumor necrosis factor-alpha gene polymorphism and death due to acute cellular rejection in a subgroup of heart transplant recipients. Hum Immunol. 2001;62(2):140–142. doi: 10.1016/s0198-8859(00)00235-4. [DOI] [PubMed] [Google Scholar]
- 14.Baan CC, Balk AH, Dijke IE, Korevaar SS, Peeters AM, de Kuiper RP, et al. Interleukin-21: an interleukin-2 dependent player in rejection processes. Transplantation. 2007;83(11):1485–1492. doi: 10.1097/01.tp.0000264998.23349.54. [DOI] [PubMed] [Google Scholar]
- 15.Dengler TJ, Zimmermann R, Braun K, Muller-Bardorff M, Zehelein J, Sack FU, et al. Elevated serum concentrations of cardiac troponin T in acute allograft rejection after human heart transplantation. Journal of the American College of Cardiology. 1998;32(2):405–412. doi: 10.1016/s0735-1097(98)00257-5. [DOI] [PubMed] [Google Scholar]
- 16.Dijke IE, Caliskan K, Korevaar SS, Maat AP, Zondervan PE, Balk AH, et al. FOXP3 mRNA expression analysis in the peripheral blood and allograft of heart transplant patients. Transplant immunology. 2008;18(3):250–254. doi: 10.1016/j.trim.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Keslar K, Rodriguez ER, Tan CD, Starling RC, Heeger PS. Complement gene expression in human cardiac allograft biopsies as a correlate of histologic grade of injury. Transplantation. 2008;86(9):1319–1321. doi: 10.1097/TP.0b013e3181889831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Villa F, Benito B, Llancaqueo M, Cuppoletti A, Roig E. Elevated levels of serum interleukin-6 are associated with low grade cellular rejection in patients with heart transplantation. Transplantation proceedings. 2006;38(9):3012–3015. doi: 10.1016/j.transproceed.2006.08.113. [DOI] [PubMed] [Google Scholar]
- 19.Melter M, Exeni A, Reinders ME, Fang JC, McMahon G, Ganz P, et al. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104(21):2558–2564. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- 20.Reinders ME, Fang JC, Wong W, Ganz P, Briscoe DM. Expression patterns of vascular endothelial growth factor in human cardiac allografts: association with rejection. Transplantation. 2003;76(1):224–230. doi: 10.1097/01.TP.0000071363.55007.D0. [DOI] [PubMed] [Google Scholar]
- 21.Seki A, Fishbein MC. Predicting the development of cardiac allograft vasculopathy. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2014;23(5):253–260. doi: 10.1016/j.carpath.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Barr ML, Cohen DJ, Benvenisty AI, Hardy M, Reemtsma K, Rose EA, et al. Effect of anti-HLA antibodies on the long-term survival of heart and kidney allografts. Transplantation proceedings. 1993;25(1 Pt 1):262–264. [PubMed] [Google Scholar]
- 23.Ciubotariu R, Liu Z, Colovai AI, Ho E, Itescu S, Ravalli S, et al. Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest. 1998;101(2):398–405. doi: 10.1172/JCI1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itescu S, Tung TC, Burke EM, Weinberg AD, Mancini D, Michler RE, et al. An immunological algorithm to predict risk of high-grade rejection in cardiac transplant recipients. Lancet. 1998;352(9124):263–270. doi: 10.1016/S0140-6736(98)09475-6. [DOI] [PubMed] [Google Scholar]
- 25.Reed EF, Hong B, Ho E, Harris PE, Weinberger J, Suciu-Foca N. Monitoring of soluble HLA alloantigens and anti-HLA antibodies identifies heart allograft recipients at risk of transplant-associated coronary artery disease. Transplantation. 1996;61(4):566–572. doi: 10.1097/00007890-199602270-00009. [DOI] [PubMed] [Google Scholar]
- 26.Poggio ED, Roddy M, Riley J, Clemente M, Hricik DE, Starling R, et al. Analysis of immune markers in human cardiac allograft recipients and association with coronary artery vasculopathy. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24(10):1606–1613. doi: 10.1016/j.healun.2004.12.110. [DOI] [PubMed] [Google Scholar]
- 27.De Vlaminck I, Valantine HA, Snyder TM, Strehl C, Cohen G, Luikart H, et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6(241) doi: 10.1126/scitranslmed.3007803. 241ra277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hidestrand M, Tomita-Mitchell A, Hidestrand PM, Oliphant A, Goetsch M, Stamm K, et al. Highly sensitive noninvasive cardiac transplant rejection monitoring using targeted quantification of donor-specific cell-free deoxyribonucleic acid. Journal of the American College of Cardiology. 2014;63(12):1224–1226. doi: 10.1016/j.jacc.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruneau S, Woda CB, Daly KP, Boneschansker L, Jain NG, Kochupurakkal N, et al. Key Features of the Intragraft Microenvironment that Determine Long-Term Survival Following Transplantation. Frontiers in immunology. 2012;3:54. doi: 10.3389/fimmu.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9(11):2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 31.Sis B, Halloran PF. Endothelial transcripts uncover a previously unknown phenotype: C4d–negative antibody-mediated rejection. Curr Opin Organ Transplant. 2010;15(1):42–48. doi: 10.1097/MOT.0b013e3283352a50. [DOI] [PubMed] [Google Scholar]
- 32.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff ‘09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10(3):464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 33.Daly KP, Seifert ME, Chandraker A, Zurakowski D, Nohria A, Givertz MM, et al. VEGF-C, VEGF-A and related angiogenesis factors as biomarkers of allograft vasculopathy in cardiac transplant recipients. J Heart Lung Transplant. 2013;32(1):120–128. doi: 10.1016/j.healun.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azimzadeh AM, Pfeiffer S, Wu GS, Schroder C, Zhou H, Zorn GL, 3rd, et al. Humoral immunity to vimentin is associated with cardiac allograft injury in nonhuman primates. Am J Transplant. 2005;5(10):2349–2359. doi: 10.1111/j.1600-6143.2005.01022.x. [DOI] [PubMed] [Google Scholar]
- 35.Barber LD, Whitelegg A, Madrigal JA, Banner NR, Rose ML. Detection of vimentin-specific autoreactive CD8+ T cells in cardiac transplant patients. Transplantation. 2004;77(10):1604–1609. doi: 10.1097/01.tp.0000129068.03900.25. [DOI] [PubMed] [Google Scholar]
- 36.Jurcevic S, Ainsworth ME, Pomerance A, Smith JD, Robinson DR, Dunn MJ, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71(7):886–892. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 37.Kalache S, Dinavahi R, Pinney S, Mehrotra A, Cunningham MW, Heeger PS. Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients. J Immunol. 2011;187(2):1023–1030. doi: 10.4049/jimmunol.1004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose ML. Role of antibodies in transplant-associated cardiac allograft vasculopathy. Z Kardiol. 2000;89(Suppl 9):IX/11–IX/15. doi: 10.1007/s003920070014. [DOI] [PubMed] [Google Scholar]
- 39.Rose ML. Role of anti-vimentin antibodies in allograft rejection. Hum Immunol. 2013;74(11):1459–1462. doi: 10.1016/j.humimm.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollander Z, Lin D, Chen V, Ng R, Wilson-McManus J, Ignaszewski A, et al. Whole blood biomarkers of acute cardiac allograft rejection: double-crossing the biopsy. Transplantation. 2010;90(12):1388–1393. doi: 10.1097/TP.0b013e3182003df6. [DOI] [PubMed] [Google Scholar]
- 41.Lin D, Cohen Freue G, Hollander Z, John Mancini GB, Sasaki M, Mui A, et al. Plasma protein biosignatures for detection of cardiac allograft vasculopathy. J Heart Lung Transplant. 2013;32(7):723–733. doi: 10.1016/j.healun.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Lin D, Hollander Z, Ng RT, Imai C, Ignaszewski A, Balshaw R, et al. Whole blood genomic biomarkers of acute cardiac allograft rejection. J Heart Lung Transplant. 2009;28(9):927–935. doi: 10.1016/j.healun.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 43.Shannon CP, Hollander Z, Wilson-McManus J, Balshaw R, Ng RT, McMaster R, et al. White blood cell differentials enrich whole blood expression data in the context of acute cardiac allograft rejection. Bioinformatics and biology insights. 2012;6:49–61. doi: 10.4137/BBI.S9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuzcu EM, Kapadia SR, Sachar R, Ziada KM, Crowe TD, Feng J, et al. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. Journal of the American College of Cardiology. 2005;45(9):1538–1542. doi: 10.1016/j.jacc.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 45.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Tuzcu EM, De Franco AC, Hobbs R, Rincon G, Bott-Silverman C, McCarthy P, et al. Prevalence and distribution of transplant coronary artery disease: insights from intravascular ultrasound imaging. J Heart Lung Transplant. 1995;14(6 Pt 2):S202–S207. [PubMed] [Google Scholar]
- 47.Tuzcu EM, Hobbs RE, Rincon G, Bott-Silverman C, De Franco AC, Robinson K, et al. Occult and frequent transmission of atherosclerotic coronary disease with cardiac transplantation. Insights from intravascular ultrasound. Circulation. 1995;91(6):1706–1713. doi: 10.1161/01.cir.91.6.1706. [DOI] [PubMed] [Google Scholar]
- 48.Kapadia SR, Ziada KM, L’Allier PL, Crowe TD, Rincon G, Hobbs RE, et al. Intravascular ultrasound imaging after cardiac transplantation: advantage of multi-vessel imaging. J Heart Lung Transplant. 2000;19(2):167–172. doi: 10.1016/s1053-2498(99)00128-x. [DOI] [PubMed] [Google Scholar]
- 49.Schoenhagen P, Sapp SK, Tuzcu EM, Magyar WA, Popovich J, Boumitri M, et al. Variability of area measurements obtained with different intravascular ultrasound catheter systems: Impact on clinical trials and a method for accurate calibration. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2003;16(3):277–284. doi: 10.1067/mje.2003.45. [DOI] [PubMed] [Google Scholar]
- 50.Ashoor I, Najafian N, Korin Y, Reed EF, Mohanakumar T, Ikle D, et al. Standardization and cross validation of alloreactive IFNgamma ELISPOT assays within the clinical trials in organ transplantation consortium. Am J Transplant. 2013;13(7):1871–1879. doi: 10.1111/ajt.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawinski D, Uribarri J, Peace D, Yao T, Wauhop P, Trzcinka P, et al. 25-OH-vitamin D deficiency and cellular alloimmunity as measured by panel of reactive T cell testing in dialysis patients. Am J Transplant. 2010;10(10):2287–2295. doi: 10.1111/j.1600-6143.2010.03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisen HJ, Kobashigawa J, Starling RC, Pauly DF, Kfoury A, Ross H, et al. Everolimus versus mycophenolate mofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013;13(5):1203–1216. doi: 10.1111/ajt.12181. [DOI] [PubMed] [Google Scholar]
- 53.Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349(9):847–858. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 54.Kobashigawa JA, Pauly DF, Starling RC, Eisen H, Ross H, Wang SS, et al. Cardiac allograft vasculopathy by intravascular ultrasound in heart transplant patients: substudy from the Everolimus versus mycophenolate mofetil randomized, multicenter trial. JACC Heart failure. 2013;1(5):389–399. doi: 10.1016/j.jchf.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Poggio ED, Augustine JJ, Clemente M, Danzig JM, Volokh N, Zand MS, et al. Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation. 2007;83(7):847–852. doi: 10.1097/01.tp.0000258730.75137.39. [DOI] [PubMed] [Google Scholar]
- 56.Poggio ED, Clemente M, Hricik DE, Heeger PS. Panel of reactive T cells as a measurement of primed cellular alloimmunity in kidney transplant candidates. J Am Soc Nephrol. 2006;17(2):564–572. doi: 10.1681/ASN.2005030293. [DOI] [PubMed] [Google Scholar]
- 57.Strom TB, Suthanthiran M. Prospects and applicability of molecular diagnosis of allograft rejection. Seminars in nephrology. 2000;20(2):103–107. [PubMed] [Google Scholar]
- 58.Strom TB, Suthanthiran M. Transcriptional profiling to assess the clinical status of kidney transplants. Nature clinical practice Nephrology. 2006;2(3):116–117. doi: 10.1038/ncpneph0115. [DOI] [PubMed] [Google Scholar]
- 59.Crespo-Leiro MG, Zuckermann A, Bara C, Mohacsi P, Schulz U, Boyle A, et al. Concordance among pathologists in the second Cardiac Allograft Rejection Gene Expression Observational Study (CARGO II) Transplantation. 2012;94(11):1172–1177. doi: 10.1097/TP.0b013e31826e19e2. [DOI] [PubMed] [Google Scholar]
- 60.Yang HM, Lai CK, Gjertson DW, Baruch-Oren T, Ra SH, Watts W, et al. Has the 2004 revision of the International Society of Heart and Lung Transplantation grading system improved the reproducibility of the diagnosis and grading of cardiac transplant rejection? Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2009;18(4):198–204. doi: 10.1016/j.carpath.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Costanzo MR, Naftel DC, Pritzker MR, Heilman JK, 3rd, Boehmer JP, Brozena SC, et al. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. J Heart Lung Transplant. 1998;17(8):744–753. [PubMed] [Google Scholar]
- 62.Keogh AM, Valantine HA, Hunt SA, Schroeder JS, McIntosh N, Oyer PE, et al. Impact of proximal or midvessel discrete coronary artery stenoses on survival after heart transplantation. J Heart Lung Transplant. 1992;11(5):892–901. [PubMed] [Google Scholar]
- 63.Kobashigawa JA, Tobis JM, Starling RC, Tuzcu EM, Smith AL, Valantine HA, et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. Journal of the American College of Cardiology. 2005;45(9):1532–1537. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 64.Mehra MR, Benza R, Deng MC, Russell S, Webber S. Surrogate markers for late cardiac allograft survival. Am J Transplant. 2004;4(7):1184–1191. doi: 10.1111/j.1600-6143.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 65.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29(7):717–727. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 66.Hartmann M, Huisman J, Bose D, Jensen LO, Schoenhagen P, Mintz GS, et al. Serial intravascular ultrasound assessment of changes in coronary atherosclerotic plaque dimensions and composition: an update. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2011;12(4):313–321. doi: 10.1093/ejechocard/jer017. [DOI] [PubMed] [Google Scholar]
- 67.Mintz GS, Maehara A. Serial intravascular ultrasound assessment of atherosclerosis progression and regression. State-of-the-art and limitations. Circulation journal : official journal of the Japanese Circulation Society. 2009;73(9):1557–1560. doi: 10.1253/circj.cj-09-0475. [DOI] [PubMed] [Google Scholar]
- 68.Keslar KS, Lin M, Zmijewska AA, Sigdel TK, Tran TQ, Ma L, et al. Multicenter evaluation of a standardized protocol for noninvasive gene expression profiling. Am J Transplant. 2013;13(7):1891–1897. doi: 10.1111/ajt.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nykanen AI, Sandelin H, Krebs R, Keranen MA, Tuuminen R, Karpanen T, et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation. 2010;121(12):1413–1422. doi: 10.1161/CIRCULATIONAHA.109.910703. [DOI] [PubMed] [Google Scholar]
- 70.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15(3):603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 71.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature medicine. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 72.Girnita DM, Brooks MM, Webber SA, Burckart GJ, Ferrell R, Zdanowicz G, et al. Genetic polymorphisms impact the risk of acute rejection in pediatric heart transplantation: a multi-institutional study. Transplantation. 2008;85(11):1632–1639. doi: 10.1097/TP.0b013e3181722edc. [DOI] [PubMed] [Google Scholar]
- 73.Torry RJ, Labarrere CA, Torry DS, Holt VJ, Faulk WP. Vascular endothelial growth factor expression in transplanted human hearts. Transplantation. 1995;60(12):1451–1457. doi: 10.1097/00007890-199560120-00014. [DOI] [PubMed] [Google Scholar]
- 74.Nykanen AI, Krebs R, Saaristo A, Turunen P, Alitalo K, Yla-Herttuala S, et al. Angiopoietin-1 protects against the development of cardiac allograft arteriosclerosis. Circulation. 2003;107(9):1308–1314. doi: 10.1161/01.cir.0000054623.35669.3f. [DOI] [PubMed] [Google Scholar]
- 75.Augustine JJ, Poggio ED, Clemente M, Aeder MI, Bodziak KA, Schulak JA, et al. Hemodialysis vintage, black ethnicity, and pretransplantation antidonor cellular immunity in kidney transplant recipients. J Am Soc Nephrol. 2007;18(5):1602–1606. doi: 10.1681/ASN.2006101105. [DOI] [PubMed] [Google Scholar]
- 76.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. Am J Transplant. 2005;5(8):1971–1975. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 77.Hricik DE, Rodriguez V, Riley J, Bryan K, Tary-Lehmann M, Greenspan N, et al. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am J Transplant. 2003;3(7):878–884. doi: 10.1034/j.1600-6143.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 78.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353(22):2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 79.Suthanthiran M, Schwartz JE, Ding R, Abecassis M, Dadhania D, Samstein B, et al. Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med. 2013;369(1):20–31. doi: 10.1056/NEJMoa1215555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hodges AM, Lyster H, McDermott A, Rice AJ, Smith JD, Rose ML, et al. Late antibody-mediated rejection after heart transplantation following the development of de novo donor-specific human leukocyte antigen antibody. Transplantation. 2012;93(6):650–656. doi: 10.1097/TP.0b013e318244f7b8. [DOI] [PubMed] [Google Scholar]
- 81.Kobashigawa J, Crespo-Leiro MG, Ensminger SM, Reichenspurner H, Angelini A, Berry G, et al. Report from a consensus conference on antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2011;30(3):252–269. doi: 10.1016/j.healun.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith JD, Banner NR, Hamour IM, Ozawa M, Goh A, Robinson D, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11(2):312–319. doi: 10.1111/j.1600-6143.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- 83.Petrakopoulou P, Kubrich M, Pehlivanli S, Meiser B, Reichart B, von Scheidt W, et al. Cytomegalovirus infection in heart transplant recipients is associated with impaired endothelial function. Circulation. 2004;110(11 Suppl 1):II207–II212. doi: 10.1161/01.CIR.0000138393.99310.1c. [DOI] [PubMed] [Google Scholar]
- 84.Sharples LD, Jackson CH, Parameshwar J, Wallwork J, Large SR. Diagnostic accuracy of coronary angiography and risk factors for post-heart-transplant cardiac allograft vasculopathy. Transplantation. 2003;76(4):679–682. doi: 10.1097/01.TP.0000071200.37399.1D. [DOI] [PubMed] [Google Scholar]
- 85.Grattan MT, Moreno-Cabral CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. Jama. 1989;261(24):3561–3566. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.