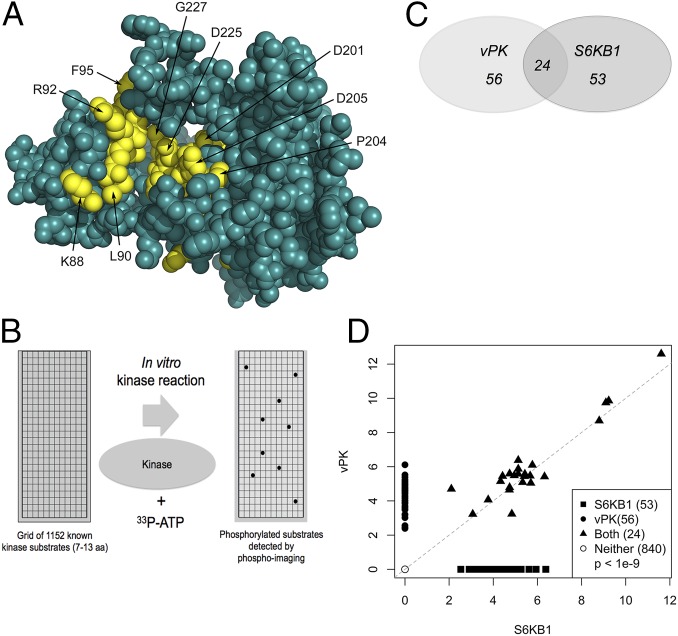
Fig. 1.
KSHV vPK displays limited homology to cellular S6KB1. (A) In silico model of vPK based on the partially activated state of S6KB1 is rendered in spheres. Residues highly conserved between S6KB1 and vPK are colored yellow, and the rest are teal. Most of the conserved residues between the two kinases occur in three motifs that cross the active site pocket. The first motif—KrLGRGaFG (uppercase residues are conserved and in yellow)—consists of residues K88 to G96. The second and third motifs—DvsPDNI and LTDFG—consist of residues D201 to I207 and L223 to G227. (B) A PepChip array was used to identify targets of both vPK and S6KB1. Recombinant kinases were incubated with radiolabeled ATP on a glass slide arrayed with >1,000 kinase substrate peptides. (C) Twenty-four peptides are phosphorylated by both vPK and S6KB1. vPK and S6KB1 were found to uniquely phosphorylate an additional 56 and 53 peptides, respectively. (D) Scatter plot analysis of spot intensities of peptides phosphorylated either uniquely by vPK (y axis; closed circles) or S6KB1 (x axis; closed squares), dually phosphorylated by both (closed triangles), or spots phosphorylated by neither kinase (open circles).