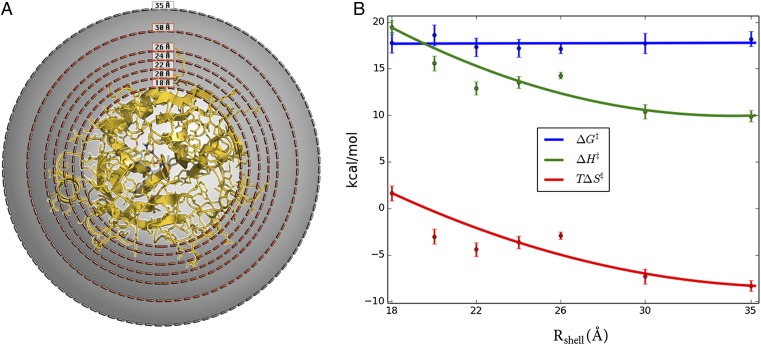
Fig. 2.
Systematic variation of protein surface flexibility and its effects on thermodynamic activation parameters. (A) Simulation sphere where solute atoms between Rshell (18, 20, 22, 24, 26, and 30 Å) and 35 Å are restrained with a force constant of 100 kcal/(mol⋅Å2). (B) Thermodynamic activation parameters (300 K) for the systems restrained in A, showing that the parameters for cold-active trypsin are gradually converted to those of the warm-adapted trypsin. See Table S1 for the number of solute atoms in each restrained shell.