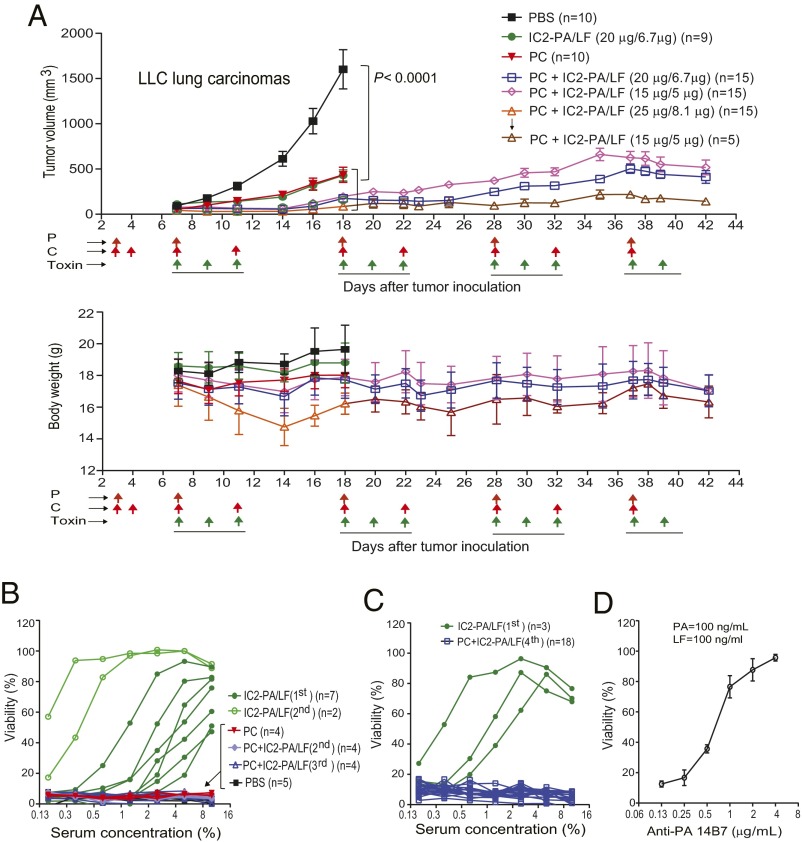
Fig. 5.
Remarkable antitumor efficacy of combined therapy with the engineered anthrax toxin and pentostatin plus cyclophosphamide regimen. (A) LLC lung carcinoma-bearing immunocompetent C57BL/6J mice were treated intraperitoneally with PBS, IC2-PA/LF (20 µg/6.7 µg), PC regimen (20 µg of pentostatin and 1 mg of cyclophosphamide), high (25 µg/8.1 µg), medium (20 µg/6.7 µg), or low (15 µg/5 µg) doses of IC2-PA/LF combined with PC regimen. Schedules for PC and the toxin treatments are indicated by the arrows. Tumor weights, mean ± SE; body weights, mean ± SD. Note that the high dose group was stopped due to deaths occurring after the first cycle treatment. The survivors in the high group were continued in the following cycles with the low dose of IC2-PA/LF. No deaths occurred in other groups during the courses of treatment. Most tumors of the toxin only and the PC only groups developed a necrotic core resulting in tumor ulceration, which required euthanization in compliance with the animal study protocol approved for the study. The subset of the IC2-PA/LF group that entered the second round therapy were shown not to respond to the treatments due to high titers of toxin-neutralizing antibody as shown in B. One-way ANOVA for tumor size differences: PBS vs. all other groups, P < 0.0001; PC plus IC2-PA/LF (20 µg/6.7 µg) vs. PC or IC2-PA/LF (20 µg/6.7 µg) (n = 9), P < 0.01. (B and C) RAW264.7 cells were incubated with PA/LF (100 ng/mL each) for 5 h in the presence of various dilutions of sera obtained from representative mice in A after the first, second, or third round of therapy (B) or after final round of therapy (fourth) (C). Cell viabilities were determined by MTT assay as described in Materials and Methods. Note that no neutralizing antibodies were detected in all of the cases from the PC and combined therapy groups. (D) As performed in B and C, 14B7 anti-PA monoclonal antibody was used as a positive control for neutralizing antibodies.