Fig. 5.
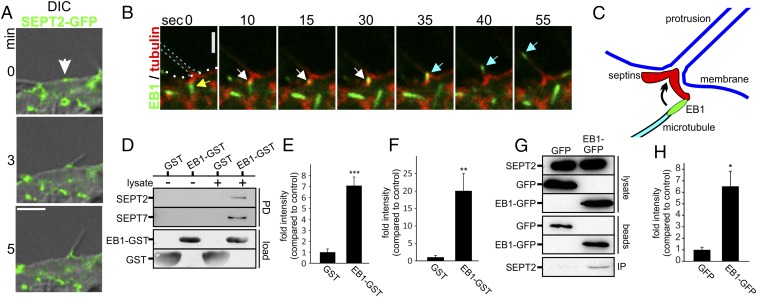
Microtubules are guided along septins. (A) Combined DIC and confocal images of Caco-2 cells. Cells were transfected with SEPT2-GFP and treated with CDT (200 ng/mL CDTa and 400 ng/mL CDTb) for 170 min (white arrow, site of protrusion formation with preexisting septin accumulation). (Scale bar, 5 µm.) (B) Caco-2 cells were transfected with SEPT6-tomato and EB3-GFP. The 55-s time-lapse sequence after CDT intoxication (as in A) for 1.5 h. Yellow arrowhead marks an EB3-positive microtubule plus-end, approaching a septin structure at the protrusion base. White arrowhead marks the same plus-end pausing at the base of protrusion. Cyan arrowhead shows redirection and fast entering into the protrusion. (C) Model for redirection of polymerizing microtubules into protrusions. Plus-ends of polymerizing microtubules contact a septin chevron-like structure and are redirected into the protrusion. (D) Representative blot of an EB1-GST pull-down of endogenous SEPT2 and SEPT7 from Caco-2 cell lysates. GST-loaded beads were used as a control. (E) Quantification of blots as in D for SEPT7. Blots were normalized to the amount of SEPT7 in the lysate. Data are ± SEM, n = 4. (F) Quantification of blots as in D for SEPT2. Blots were normalized to the amount of SEPT2 in the lysate. Data are ± SEM and n = 4. (G) Representative blot of a coimmunopreciptitation of SEPT2 along with EB1-GFP. HeLa cells were transfected with EB1-GFP for 24 h. SEPT2 and EB1-GFP from the lysates were coimmunoprecipitated with anti-GFP antibody conjugated to magnetic beads. GFP-transfected cells were used as control. (H) Quantification of blots as in G for SEPT2. Blots were normalized to the amount of EB1-GFP and GFP on beads. Data are ± SEM and n = 3.