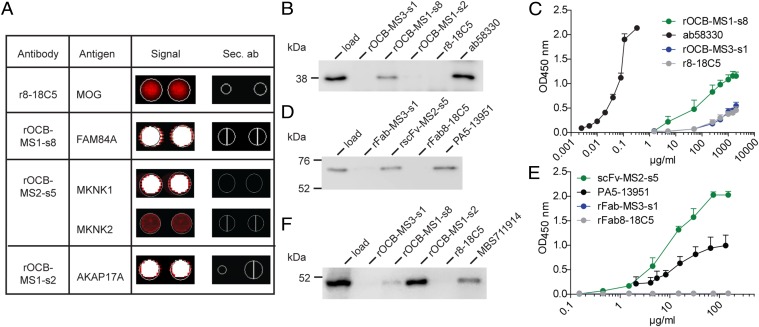
Fig. 4.
Identification and validation of antigens of OCB antibodies from patients with MS. (A) Hybridization of the control antibody r8-18C5 and three rOCB antibodies to ProtoArrays. Spots are shown in duplicate. The color code ranges from black (no reactivity) to red (medium reactivity) and white (strong reactivity). Compilation of rOCBs (left column), their antigens (second column), the signals from array hybridization (third column), and the corresponding signals from hybridization of secondary antibodies alone (fourth column) is shown. The anti-MOG antibody r8-18C5 showed an intermediate, but specific, signal with MOG (second row); rOCB-MS1-s8 showed strong signal with FAM84A (third row); rOCB-MS2-s5 showed a strong signal with MKNK1 and an intermediate signal with the highly homologous isoform MKNK2 (fourth and fifth rows); and rOCB-MS1-s2 showed strong signals with AKAP17A (sixth row). All secondary antibodies alone (sec. Ab) showed no reactivity (fourth column). (B) Validation of FAM84A recognition by rOCB-MS1-s8 by immunoprecipitation. Recombinant FAM84A produced in HEK293 cells was specifically precipitated by rOCB-MS1-s8 and the commercial anti-FAM84A antibody ab58330, but not by control antibodies rOCB-MS3-s1, rOCB-MS1-s2, and r8-18C5. Lane 1 shows the loading control. The blot is representative of three independent experiments. (C) Titration of FAM84A recognition by rOCB-MS1-s8 analyzed by ELISA. Recombinant FAM84A produced in E. coli was specifically recognized by rOCB-MS1-s8 in a dose-dependent manner. The commercial monoclonal anti-FAM84A antibody ab58330 recognized FAM84A at considerably lower concentrations, indicating higher avidity. The negative control antibodies rOCB-MS3-s8 and r8-18C5 bound only very weakly at very high concentrations. The secondary antibody alone yielded background signal. Error bars indicate SD of the mean. Data are representative of four independent experiments. (D) Validation of MKNK1 recognition by rOCB-MS2-s5 by immunoprecipitation. Recombinant MKNK1 produced in HEK293 cells was specifically precipitated by rOCB-MS2-s5 and the commercial anti-MKNK1 antibody PA5-13951, but not by control antibodies rOCB-MS3-s1 and r8-18C5. Lane 1 shows the loading control. The blot is representative of three independent experiments. (E) Titration of MKNK1 recognition by rscFv-MS2-s5 analyzed by ELISA. Recombinant MKNK1 produced in HEK293 cells was specifically recognized by rscFv-MS2-s5 and with a slightly higher avidity by the commercial anti-MKNK antibody PA5-13951, but not by control Fab fragments rFab-MS3-s1 and rFab-8-18C5. Error bars indicate SD of the mean. Data are representative of four independent experiments. (F) Validation of AKAP17A recognition by rOCB-MS1-s2 by immunoprecipitation. AKAP17A produced in HEK293 EBNA cells was precipitated by rOCB-MS1-s2 and -s8 and the commercial anti-AKAP17A antibody MBS711914, but not by the control antibodies rOCB-MS3-s1 and r8-18C5. Lane 1 shows the loading control. The blot is representative of five independent experiments.