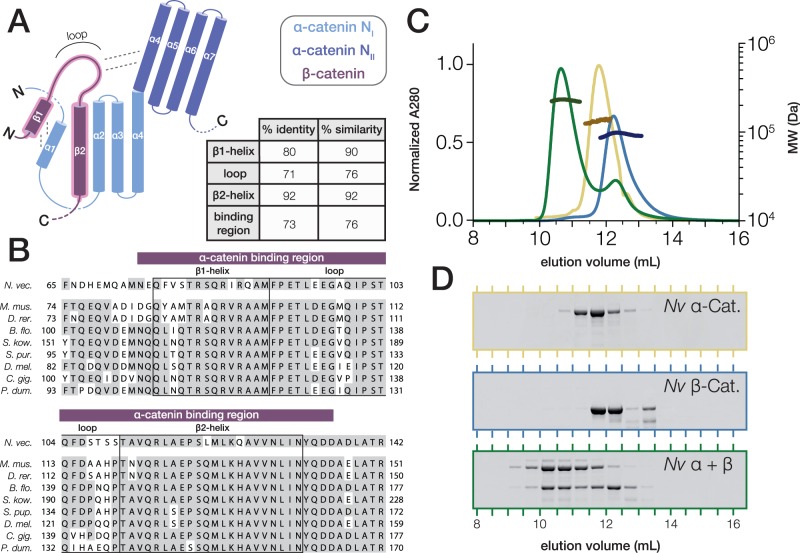
Fig. 3.
Nematostella vectensis α- and β-catenin bind to form a heterodimer. (A) Schematic of the α-catenin·β-catenin complex based on crystal structures of mammalian orthologs. The interacting surface is highlighted (red). β-catenin helices β1 and β2 displace the N-terminal α-catenin α1 helix from the NI domain in order to form 2 four-helix bundles with α-catenin helices α1–α4 (Pokutta et al. 2014). The loop connecting β-catenin helices β1 and β2 interacts with the NII bundle of α-catenin. Percent Identity and similarity between N. vectensis β-catenin and a bilaterian consensus sequence is compared for the interacting β1 and β2 helices and loop region, and the entire binding region. (B) A multiple alignment of the α-catenin binding region of N. vectensis β-catenin with the corresponding region of β-catenin orthologs from representative bilaterian species. β1 and β2 helices and loop region (boxes), as well as the entire binding region (red bar), are annotated. Abbreviations used for species names are as follows: B. flo.—B. floridae; C. gig.—C. gigas; D. mel.—D. melanogaster; D.rer.—D. rerio; M. mus.—M. musculus; N. vec.—N. vectensis; P. dum.—P. dumerilii; S. kow.—S. kowalevskii; S. pur.—S. purpuratus. (C) SEC-MALS elution profiles for N. vectensis α- and β-catenin (yellow and blue, respectively) run independently, and following co-incubation at 18 °C for 30 min (green). MW measurements corresponding to the UV peaks for each run are plotted in darker shades of the same color. (D) SDS–PAGE of fractions collected from (C).