Fig. 5.
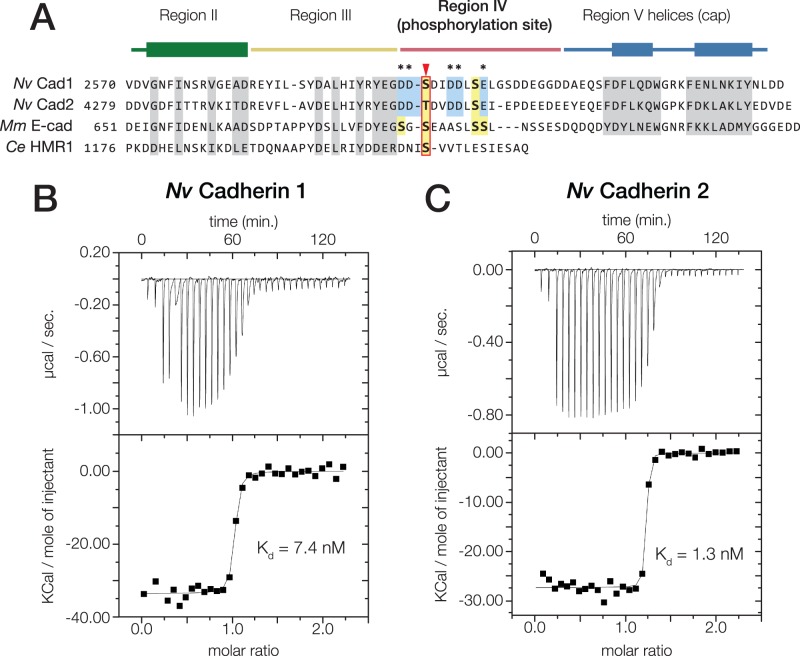
Nematostella vectensis Cadherin-1 and -2 bind to N. vectensis β-catenin with high affinity. (A) Alignment of the cytoplasmic domain of N. vectensis Cadherin-1 and -2 with those of M. musculus E-cadherin and C. elegans HMR1. Conserved residues shown to be critical for the mammalian E-cadherin·β-catenin interaction are highlighted (grey). Regions II–V as identified in (Huber and Weis 2001) are annotated above with colored bars; the thickened rectangles indicate helices. Known phosphorylated residues in mammalian E-cadherin are highlighted (yellow boxes), and the conserved phosphoserine residue affecting β-catenin affinity is indicated (red arrow). Acidic residues within the phosphorylation site of N. vectensis cadherins that are not present in mammalian E-cadherin are annotated (blue boxes, asterisks). (B and C) Nematostella vectensis Cadherin-1 and -2 binding to N. vectensis β-catenin was quantified using ITC. (B) Nematostella vectensis Cadherin-1 was titrated into N. vectensis β-catenin. The ratio of heat released (Kcal) per mole of N. vectensis Cadherin-1 injected into N. vectensis β-catenin was plotted against the molar ratio of the two proteins, and the Kd calculated from these measurements is indicated. (C) Nematostella vectensis Cadherin-2 was titrated into N. vectensis β-catenin as in (B).