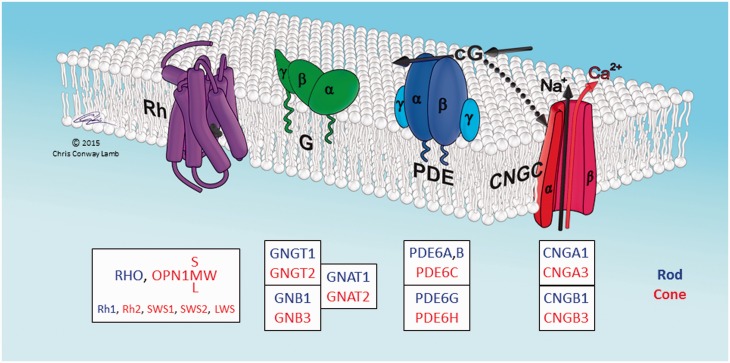
Fig. 1.
Schematic of proteins involved in activation of vertebrate phototransduction. The four principal proteins involved in activation of the light response in vertebrate photoreceptors are depicted schematically. The cytoplasmic surface of the lipid membrane is shown uppermost, and the illustrated arrangement is for the sac/plasma membrane of cone photoreceptors; in rods the first three proteins (Rh, G, PDE) are located in the disc membrane that has become pinched-off from the plasma membrane. Rh: rhodopsin, or its cone equivalent. G: heterotrimeric G-protein, transducin. PDE: tetrameric cGMP phosphodiesterase, PDE6. CNGC: tetrameric cyclic nucleotide-gated ion channel. cG: cytoplasmic messenger, cGMP. Boxes list the HGNC gene names of the human isoforms. Blue denotes isoforms expressed primarily in rods, and red denotes isoforms expressed primarily in cones. Lower line in the Rh box lists the names used for the five isoforms found in nonmammalian vertebrates. When Rh absorbs a photon, its retinaldehyde ligand is isomerized from its 11-cis configuration to the all-trans isomer, which triggers a structural change in the protein, converting Rh to its active form. The single molecule of activated Rh sequentially activates numerous molecules of transducin, G. The α subunit of each activated transducin, Gα, then partly activates a molecule of PDE, by binding to its inhibitory γ subunit, thereby relieving the inhibition; for full activation of the PDE, both γ subunits need to have bound a molecule of Gα. The activated PDE hydrolyzes the cytoplasmic messenger cGMP, and the resulting reduction in cytoplasmic cGMP concentration causes closure of the CNGC ion channels. This reduces the influx of cations into the cell, making the interior more negative (hyperpolarization) and thereby generating the photoreceptor’s electrical response to light. For a description of the proteins, see Wensel (2008), and for a quantitative description of the activation steps, see Lamb and Pugh (1992). Illustration © Chris Conway Lamb, with permission.