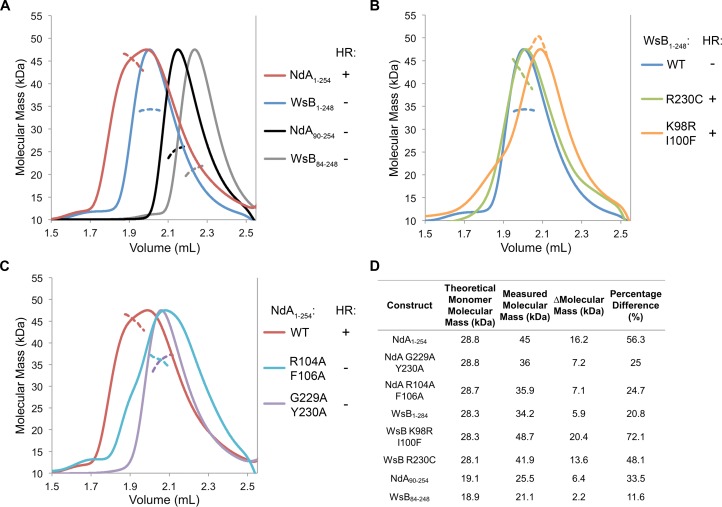
Fig 2. The autoactivity of the RPP1 N-TIR domain is correlated with self-association in solution.
Purified (N-)TIR domain proteins from the Niederzenz (NdA) and Wassilewskija (WsB) alleles of RPP1 were analyzed by size-exclusion chromatography (SEC) coupled with multi-angle laser light scattering (MALS). For each sample, 175 μg of purified protein was separated on a Superdex Increase 200 5/150 GL SEC column and the molecular mass calculated across the elution peak. The colored solid line represents the normalized refractive index trace (arbitrary units) of the protein eluting from the SEC column. At the elution peak, the averaged molecular mass (kDa) of the proteins was calculated from the protein concentration (derived from the refractive index changes) and light scattering data. The averaged molecular masses across the elution peak are represented by dashed lines of the corresponding color. In planta hypersensitive response (HR) phenotypes are indicated for each construct by a “+” (autoactive) or “-”(non-autoactive). These phenotypes are documented in Fig 1, S2 and S4 Figs. (A) Comparison of the solution properties of RPP1 TIR domains with and without native RPP1 N termini. Numbers in the legend refer to the amino acids that comprise each protein sample. (B) Solution properties of the wild-type WsB N-TIR domain and the gain-of-autoactivity mutants, WsB R230C and WsB K98R I100F. (C) Solution properties of the wild-type NdA N-TIR domain and the loss-of-autoactivity mutants, NdA G229A Y230A and NdA R104A F106A. (D) Comparison of theoretical monomer molecular masses and the measured molecular masses for the proteins analyzed in (A-C).