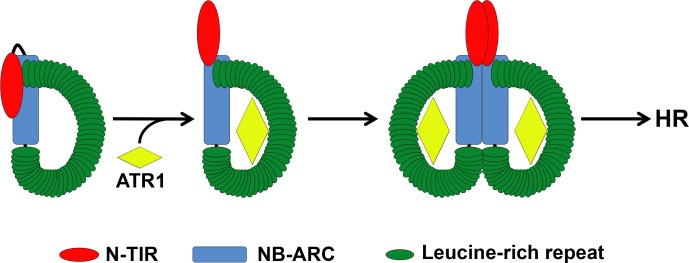
Fig 9. Proposed model of cell death activation by RPP1.
In the absence of ATR1, RPP1 is likely maintained in a largely inactive state by a network of N-TIR:NB, NB:LRR, and N-TIR:LRR interactions. Note that the N-TIR:NB interaction occurs at the putative oligomerization interface to prevent effector-independent association. Binding of a recognized allele of ATR1 to the LRR domain may stabilize conformational transitions that reorient the N-TIR domain to expose the oligomerization interface. This allows RPP1 oligomerization via the NB domain, potentially stabilized by LRR:LRR interactions. The reorientation of the N-TIR domain also permits N-TIR domain self-association, which outcompetes N-TIR:NB interactions and ultimately triggers a cell death response (HR). While this model accounts for the effector-independent NB:LRR interaction, the specific interaction interface(s) could differ before and after RPP1 activation.