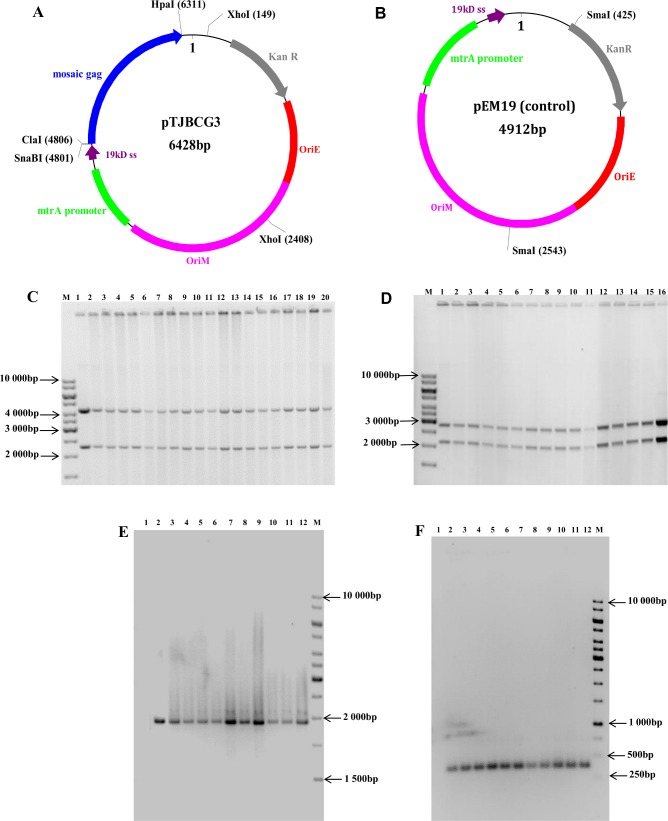
Fig 2. Schematic representation of the BCG shuttle vectors and the determination of their genetic integrity before and after vaccination.
Schematic representation of (A) pTJBCG3, which was used to make the BCG-GagM vaccine, (B) pEM19 which was used to make the BCGE vaccine. Restriction sites used for cloning and restriction mapping analysis are indicated in black bold type. OriE–E. coli origin of replication; OriM–mycobacterial origin of replication, 19kD ss– 19kD signal sequence; KanR–kanamycin resistance gene. (C) pTJBCG3 digested with XhoI. Lane 1 is a positive control of pTJBCG3 DNA prior to transformation into BCGΔpanCD. Lanes 2–21 contain pTJBCG3 DNA obtained from recombinant BCG-GagM vaccine stocks. (D) pEM19 digested with SmaI. Lanes 1–15 contain pEM19 plasmid DNA isolated from recombinant BCGE vaccine stocks, and lane 16 pEM19 plasmid DNA isolated prior to transformation into BCGΔpanCD (positive control). PCR amplification of DNA from rBCG obtained from the spleens and lymph nodes of mice vaccinated with BCG-GagM (Group 2) (E) or BCGE (Group 5) (F) 11.5 weeks post vaccination. Lane 1 –negative control; Lane 2 –positive control; Lane 3–12 are PCR products from rBCG isolated from homogenised spleen (3–7) or lymph nodes (8–12). Lanes M in C—F contain the molecular weight marker O’GeneRulerTM 1kb DNA ladder.