Abstract
The aim of this work was to explore the ability of free arachidonic acid, palmitic acid and the unsaturated fatty acids oleic acid and docosahexaenoic acid to modify calcium homeostasis and mitochondrial function in rat pachytene spermatocytes and round spermatids. In contrast to palmitic acid, unsaturated fatty acids produced significant increases in intracellular calcium concentrations ([Ca2+]i) in both cell types. Increases were fatty acid specific, dose-dependent and different for each cell type. The arachidonic acid effects on [Ca2+]i were higher in spermatids than in spermatocytes and persisted when residual extracellular Ca2+ was chelated by EGTA, indicating that the increase in [Ca2+]i originated from release of intracellular calcium stores. At the concentrations required for these increases, unsaturated fatty acids produced no significant changes in the plasma membrane potential of or non-specific permeability in spermatogenic cells. For the case of arachidonic acid, the [Ca2+]i increases were not caused by its metabolic conversion to eicosanoids or anandamide; thus we attribute this effect to the fatty acid itself. As estimated with fluorescent probes, unsaturated fatty acids did not affect the intracellular pH but were able to induce a progressive decrease in the mitochondrial membrane potential. The association of this decrease with reduced reactive oxygen species (ROS) production strongly suggests that unsaturated fatty acids induced mitochondrial uncoupling. This effect was stronger in spermatids than in spermatocytes. As a late event, arachidonic acid induced caspase 3 activation in a dose-dependent manner both in the absence and presence of external Ca2+. The concurrent but differential effects of unsaturated fatty acids on [Ca2+]i and mitochondrial functions are additional manifestations of the metabolic changes that germ cells undergo during their differentiation.
Introduction
The functional relationship between germ cells and Sertoli cells (SC) in the mammalian seminiferous tubules occurs either through juxtacrine signalling (adhesion molecules) or paracrine signalling (molecules secreted into the extracellular space of the adluminal compartment) [1]. However, the precise composition of the extracellular environment of germinal cells in the seminiferous tubules has not been determined. Glucose gains access to the luminal compartment through SC [2], which also secrete lactate to the luminal and adluminal compartments as a product of SC glucose metabolism [3–5]. Since the glycolytic activity of SC is stimulated by follicle stimulating hormone (FSH), β-adrenergic agonists, interleukin 1 β (IL1-β) and tumour necrosis factor α (TNF-α) [4–7], a similar, though inverse, regulation of lactate and glucose secretion toward the adluminal compartment is expected. Lactate and glucose have been shown to modulate [Ca2+]i, intracellular pH (pHi) and mechanisms of cell death in spermatogenic cells [8,9].
SC also produce arachidonic acid (AA) and some of its metabolites in an FSH-regulated manner [10]. In a mouse SC-derived cell line (TM4), activation of CD95 (Fas) [11,12] activates cytosolic phospholipase A2 (PLA2) and AA release [13]. Likely via an autocrine loop, AA activates glycolytic lactate production in SC [14]. Accumulated evidence strongly suggests that AA is part of the regulatory signalling networks regulating spermatogenesis. In support of this idea, a diet rich in AA has a large impact on animal fertility [15] and mice lacking the group VI PLA2 isoform show impaired sperm motility and greatly reduced fertility [16].
Unsaturated fatty acids (UFAs) are known to mobilize [Ca2+]i in pancreatic β cells and neutrophils [17,18]. The importance of [Ca2+]i homeostasis in spermatogenic progression is strongly suggested by the fact that a blockade of ryanodine receptors reduces spermatogonia proliferation and induces meiosis in spermatocytes [19]. Additional evidence in support of a role for [Ca2+]i mobilization in germ cells is that 1) Ca2+ entry is involved in expression of the antiapoptotic proteins Bcl-xS and Bcl-xL [20]; 2) mice deficient in CIB1, a calcium binding protein, show increased apoptosis in spermatogenic cells [21]; 3) voltage-activated calcium channel blockers induce arrest of spermatogenesis at the elongating spermatid level in mice [22] and 4) genetic ablation of CAMK4 (calmodulin dependent kinase) impairs spermatogenesis [23]. The aim of this work was to examine whether free fatty acids regulate [Ca2+]i homeostasis of pachytene spermatocytes and round spermatids. We chose two polyunsaturated FA, AA and docosahexaenoic acids (DHA) as well as oleic acid (OA) and palmitic acid (PA) because they are abundant in membrane glycerophospholipids of all cells in the seminiferous epithelium [24,25].
Materials and Methods
Animals
Adult (40–60 days old) male Sprague–Dawley rats were acquired from the Animal Facility in the Faculty of Biological Sciencies Pontifical Catholic University of Chile. The rats were housed under a 12L:12D cycle with water and rat chow ad libitum and were euthanized by narcotization with CO2 followed by cervical dislocation.
Ethical statement
All the experiments were conducted in accordance with the guidelines outlined by the Consortium for Development in the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching and by the National Research Council. All experimental protocols were reviewed and approved by the Chilean National Fund for Science and Technology (FONDECYT) and the Ethics Committee of the Pontificia Universidad Catolica de Valparaíso (EC-PUCV-10/2013). None of the authors served on this committee.
Chemicals
Fura-2 acetoxymethyl ester; 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF); bis-(1,3-dibutylbarbituric acid) trimethine oxonol (oxonol), 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA; reactive oxygen and nitrogen species (ROS/RNS) sensor) and 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1), were purchased from Molecular Probes (Life Technologies, USA). Arachidonic, oleic, docosahexanoic, palmitic acid, indomethacin (inhibitor of cyclooxygenase, Indomet), nordihydroguaiaretic acid (inhibitor of lipoxygenase, NDGA), valinomycin, luciferin, luciferase, rhodamine 123, salts and buffers were obtained from Sigma-Aldrich (St. Louis, MO, USA). 2-(1-thienyl)ethyl 3,4-dihydroxybenzylidenecyanoacetate (inhibitor of lipoxygenase, 2-TEDC), was purchased from Tocris Bioscience (through Gene-Xpress, Chile).
Indomethacin, NDGA and 2-TEDC have been shown to be cell permeant and have 50% inhibitory concentrations of 0.1–0.2 μM for indomethacin [26], 0.5–1.0 μM for NDGA [27] and 0.09, 0.013 and 0.5 μM (5-, 12-, and 15-lipoxygenases, respectively) for 2-TEDC [28].
Experimental procedure
Adult rats were maintained in groups of three to four animals per cage. Groups of two to three rats were chosen at random and used to isolate pachytene spermatocytes and round spermatids. Rats were euthanized in the Animal Facility, and the testes of each rat were isolated in a room specially equipped for this procedure.
Rat pachytene spermatocytes and round spermatid isolation
Rat spermatogenic cell populations were isolated using velocity sedimentation separation through a 2–4% bovine serum albumin (BSA) gradient, as described [29]. The pachytene spermatocyte (85 ± 5% purity) and round spermatid fractions (92 ± 4% purity) were identified both by their size as well as by the typical nuclear feature after staining with Hoechst 33342 [30]. Unless otherwise stated, measurements of cell suspensions of pachytene spermatocytes or round spermatids labelled with specific fluorescent probes were collected using a Fluoromax 2 fluorimeter (Jobin-Yvon-Spex, NJ, USA).
[Ca2+]i measurements of rat spermatogenic cells in suspension
Rat spermatogenic cells were resuspended (20 x 106 cells/mL) in KH media (140 mM NaCl, 4 mM KCl, 1.6 mM MgCl2, 1.6 mM KH2PO4, 10 mM HEPES, pH 7.4) containing or lacking Ca2+ (0.5 mM CaCl2 or 1 mM EGTA, respectively). Metabolic substrates for the cells were either 5 mM L-Lactate (KH-lactate) or 5 mM glucose (KH-glucose). The cells in KH-lactate media were loaded with 5 μM of the calcium probe Fura-2 AM by incubation for 1 hr at room temperature in an oxygenated atmosphere followed by three washes at 4°C and resuspension in the same media The fluorescence measurements were performed by adding a concentrated cell suspension (50 μL) to a temperature-regulated spectrofluorimeter cuvette (3.0 mL, with stirring) containing 2.5 mL of one of the four different types of media described above to which fatty acids have previously been added. The [Ca2+]i determinations were carried out using a ratiometric method as described [31]. Fura-2 calibration was performed by cell lysis with digitonin (20–25 μg/mL) and addition of 1 mM EGTA to determine Fmin or addition of 3 mM CaCl2 to determine Fmax.
Measurements described in this work were made without the addition of external Ca2+ and 1 mM EGTA, unless otherwise indicated. The initial rates of [Ca2+]i changes induced by FAs were estimated by linear regression of the [Ca2+]i values taken between 10s and 110 s after FAs addition.
Viability assays
The percentage of cells maintaining membrane integrity was visualized using a permeant nuclear fluorescent probe (Hoechst 33342) and a non-permeable probe (propidium iodide, PI). As a second criterion, the release of a cytosolic enzyme (lactate dehydrogenase, LDH) was quantified as the decrease in NADH concentration after addition of the spermatogenic cell extracellular medium to a solution buffered to pH 7.5 with 20 mM Tris-HCl and containing 0.2 mM NADH and 5 mM pyruvate.
Mitochondrial membrane potential changes measured with rhodamine 123 fluorescence
The mitochondrial membrane potential changes induced by FA were measured using the lipophilic, cationic probe rhodamine 123 (R123). Potential-dependent accumulation of this probe in energized mitochondria results in a relatively weak fluorescence signal due to self-quenching. Dissipation of the normally negative mitochondrial membrane potential (e.g., by an uncoupler) is marked by an increase in fluorescence due to distribution of the dye throughout the cell, which results in a green shift in the excitation spectra. The fluorescence with excitation ratio 520/490 and an emission wavelength of 530 nm reflects the mitochondrial membrane potential and decreases upon depolarization of the membrane [32,33]. Spermatogenic cells were resuspended (2 x106 cells/mL) in High K+-media (HK+) that contained no nominal calcium (5 NaCl mM, 140 KCl mM, 1.5 mM MgCl2, 10 mM KH2PO4, 10 mM Hepes, 1 mM EGTA, 0.2 mM EDTA, pH 7.4; supplemented with 10 mM lactate). Membranes were permeabilized with digitonin using 20 and 25 μg/mL for spermatids and pachytene spermatocytes, respectively [34], and exposed to 300 nM of R123 for 5 min. Subsequently, the excitation ratio (520/490 nm) at an emission wavelength of 530 nm was measured at 33°C as a function of time.
The JC-1 dye was also used to evaluate FA-induced mitochondrial membrane potential changes. Accumulation of this probe in active mitochondria is sensitive to the membrane potential, and the fluorescence emission shifts from green to red due to the formation of red fluorescent J-aggregates. Mitochondrial depolarization is indicated by a decrease in the red/green fluorescence intensity ratio. The emission ratio 590/560 with an excitation wavelength of 514 nm reflects the mitochondrial membrane potential and is expected to decrease upon depolarization [35]. For fluorimetric measurements, 200 nM JC-1 was incubated for 15 min with spermatogenic cells resuspended (1 x 106 cells/mL) in KH-lactate media supplemented with 0.5 mM CaCl2. Subsequently, vehicle (< 2 μL ethanol) or the fatty acids were added.
Membrane potential measurements using bis-(l,3 diethyl)thiobarbiturate trimethine oxonol
Changes in plasma membrane potential were determined using the negatively charged, permeant fluorescent probe oxonol with an excitation at 535 nm and an emission at 560 nm. After measuring the baseline fluorescence of 500 nM oxonol in a 3 mL cuvette kept at 33°C, 50 μL of a concentrated cell suspension was added to reach a final cell concentration 2 x 106 cells/mL. After stabilization of the fluorescence at 33°C, the FAs were added to the cuvette to the specified final concentrations and fluorescence levels were allowed to equilibrate. Finally, fluorescence was measured following the addition of valinomycin at a final concentration of 0.5 μM, which has been previously shown to hyperpolarize round spermatids [36]. In the absence of cells, changes in oxonol fluorescence were not observed for any of the FA concentrations examined in this study.
Intracellular pH measurements
The pHi of spermatogenic cells was estimated from fluorescence levels of intracellular BCECF. Cells at a density of 2 x 106 cells/mL were loaded with BCECF by incubation in KH-Ca2+-lactate media with 2 μM acetoxymethy1 BCECF for 30 min at 33°C in 95% O2/5% CO2. The measurements were performed in a Fluoromax-2 fluorometer (Jobin Ivon-Spex, USA) at an excitation fluorescence ratio of 505/445 and a 530 nm emission wavelength. Observation of the cells by epifluorescence microscopy showed a homogeneous distribution of the dye throughout the cytoplasm.
Intracellular BCECF was calibrated in HK+ media with 1 μM nigericin by measuring the excitation fluorescence ratio of 505/445 nm at different extracellular pH values [37]. The pH of the extracellular saline was varied between pH 5.5 and 8.0. Fluorescence ratios were obtained at each calibration pH and a standard curve was generated using Microcal Origin version 7.0 (Microcal Software, Inc., MA, USA). Intracellular pH values were calculated from the experimental fluorescence ratios obtained before and after addition of FA to the cells.
ATP release assay
The release of ATP from pachytene spermatocytes or round spermatids into the extracellular milieu was estimated using a luciferin-luciferase assay [38]. Measurements were taken with the fluorimeter lamp off, the excitation shutter closed, a wide emission slit width centred at 550 nm and 4 s integration time. Successive additions of cells, luciferin, luciferase, AA and digitonin were performed to test for intrinsic cell luminescence, possible residual ATP in the luciferin-luciferase reagent and the effect of AA. Release of cellular ATP upon addition of digitonin was measured as a positive control.
ROS/RNS production measurements
Rat spermatogenic cells were resuspended in KH-Ca2+-lactate media to a final concentration of 6 x 105 cells/mL. Cells were pre-incubated for 3 min at 33°C and then H2DCFDA (2.5 μM final concentration) was added to the cell suspension. Fluorescence measurements with an excitation at 495 nm and emission at 529 nm were made of dichlorofluorescein (DCF) (which is the product of H2DCFDA oxidation) at 15 s intervals for 15 min in an Appliskan multiwell fluorescence plate reader (Thermo Fisher Scientific, Inc.). Standardization of the probe and data analysis was performed as described by [39].
Caspase 3 activity measurements
Pachytene spermatocytes or round spermatids were prepared at 1.5–2 x 106 cells/mL in KH-Ca2+-lactate media. Each well of a 96 multi-well plate received 200 μL of the resulting cell suspension. To each well, either the oxidative stress and apoptosis inducer tert-butyl hydroperoxide (tBHP, 1 mM stock in DMSO) was added to a final concentration of 200 μM or AA (1 mM stock in ethanol) was added to different final concentrations and homogenized in the wells. Finally, the caspase-3 fluorogenic substrate IV (#264150, Calbiochem-Merck Millipore, Chile) was added to a final concentration of 30 μM (5 mM stock in DMSO). Measurements with an excitation at 430 nm and an emission at 480 nm were performed at 10 min intervals for 240 min.
Data and statistical analysis
The initial rate of FA-induced Ca2+ entry was determined by linear regression of the [Ca2+]i vs. time data between 30 and 200 s after FA addition. Statistical differences between treatment and control rates of increase in [Ca2+]i were analysed by applying a t-test with Wellch’s correction or an ANOVA followed by a post-test when different experimental conditions were compared. When the data was expressed as a percentage increase in [Ca2+]i by each FA or when the F-test indicated significant differences in group variances, the comparison between treatment and control was performed using non-parametrical Mann-Whitney analysis [40]. In all cases, measurements were carried out from samples derived from at least two cell preparations with four testicles each (two rats). The results were expressed as the mean ± standard error of the mean (SEM).
Results
Dose-response curves of [Ca2+]i changes induced by FA in spermatogenic cells
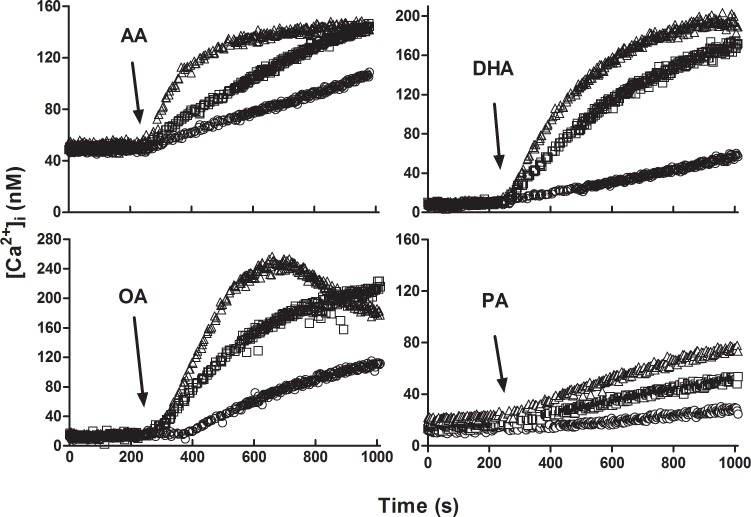
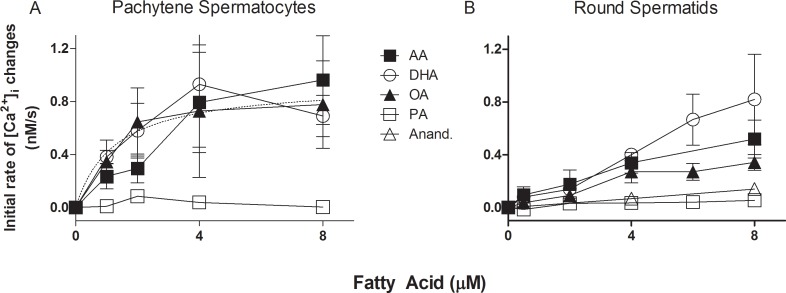
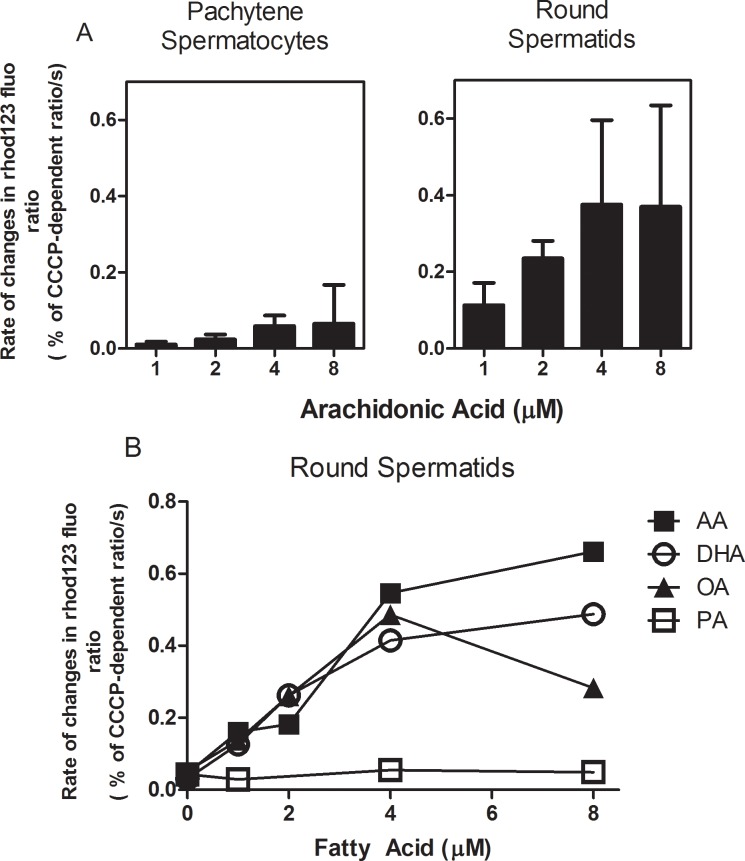
Fig 1 shows the kinetics of [Ca2+]i after addition of arachidonic (AA), docosahexaenoic (DHA), oleic (OA) and palmitic acid (PA) in round spermatids. [Ca2+]i changes induced by FA in KH-lactate-EGTA media were dose-dependent. At 8 μM, OA showed a biphasic curve that was not studied further and could be interpreted either as Ca2+ reuptake into intracellular calcium stores (ICaS) or as Ca2+ exit from the cells after 700 s of OA addition. The rates of increase in [Ca2+]i induced by different concentrations of AA, DHA, OA and PA in pachytene spermatocytes and round spermatids are compared in Fig 2. The three UFAs, but not PA, show a clear increase in [Ca2+]i in pachytene spermatocytes (Fig 2A) and round spermatids (Fig 2B). An interesting difference between the [Ca2+]i rise among these cell types was that spermatocytes showed a clearer trend toward saturation of the FA effect on [Ca2+]i, with a K0.5 of approximately 1.3 μM.
Fig 1. Time course of FA-induced [Ca2+]i changes in round spermatids in the nominal absence of external Ca2+.
After loading the cells with Fura-2, 2 (○), 4 (□) or 8 μM (Δ) of each fatty acid (AA, DHA, OA and PA) was added to a suspension of round spermatids in KH-lactate-EGTA at the times indicated by arrows.
Fig 2. Concentration dependence of AA-induced changes in [Ca2+]i.
(A) Dose response curves for the rate of [Ca2+]i changes (nM/s) at different AA, DHA, OA, and PA total concentrations in suspensions of pachytene spermatocytes. The dotted line represents a hyperbolic fit of the DHA data. (B) Dose response curves for the rate of [Ca2+]i changes at different AA, DHA, OA, and PA total concentrations in suspensions of round spermatids. Similar concentrations of anandamide were included for comparison. These measurements were performed in the absence of external Ca2+.
External Ca2+ and metabolic substrate dependence of the AA-induced effects in spermatogenic cell [Ca2+]i
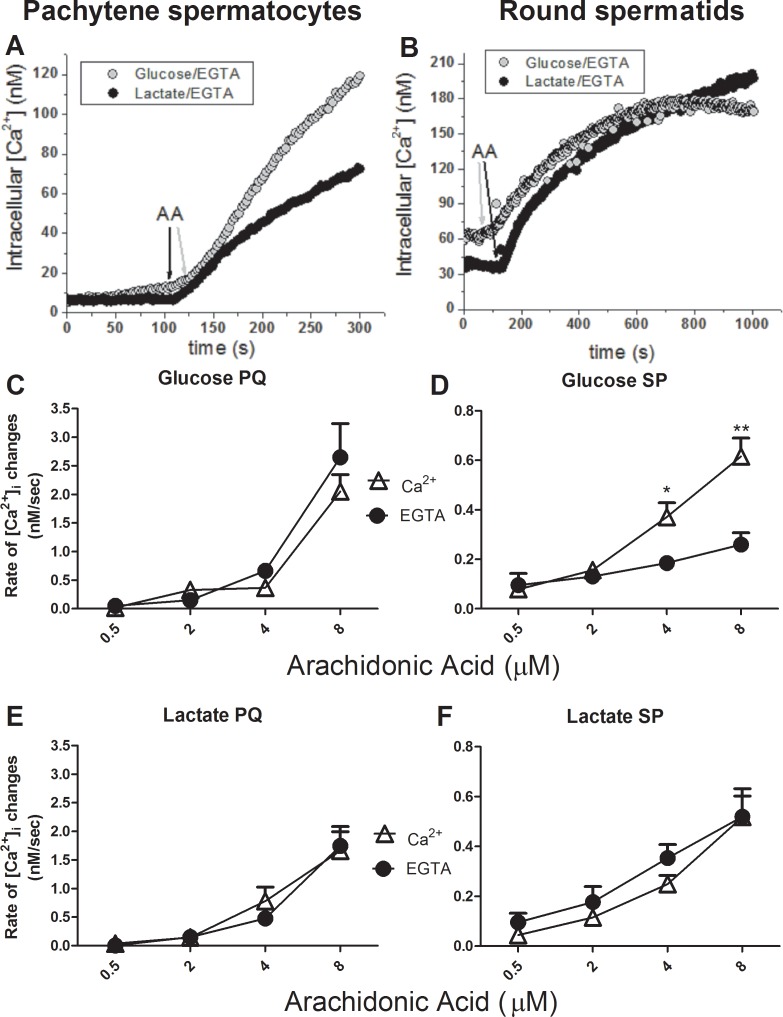
Because it was previously shown that Ca2+ homeostasis in isolated spermatogenic cells was dependent on which metabolic substrate was present in the media [41], we first tested the influence of AA on the [Ca2+]i in pachytene spermatocytes or round spermatids incubated with glucose or lactate as metabolic substrates. In the presence of external lactate (Fig 3A and 3B), in both cell types there was an increase in initial rates of [Ca2+]i changes that depended upon the external AA concentration but not the presence of external calcium. This extracellular calcium independence strongly suggests that AA was able to induce the release of Ca2+ from ICaS. A significant difference emerged in the presence of glucose, where the initial rise of [Ca2+]i induced by AA was significantly higher in calcium-containing rather than in calcium-free media in round spermatids (Fig 3A). This difference is most likely due to the ability of glucose to release Ca2+ from the ICaS in round spermatids [8,41], a phenomenon associated to the ability of glucose to decrease intracellular [ATP] in round spermatids and a possible entry of external Ca2+ by activation of store-operated Ca2+ channels (Darszon et al., 2012). The glucose-dependent effect on initial rate of AA-induced [Ca2+]i rise observed in round spermatids was not observed in pachytene spermatocytes, which is in agreement with the known metabolic properties of these two cell types and their differences in [Ca2+]i homeostasis [42].
Fig 3. Metabolic substrates and external Ca2+ effects on AA-induced changes in [Ca2+]i.
Dose response curves depicting the rate of [Ca2+]i change (nM/s) vs. [AA] in the presence and absence of external Ca2+ and with 5 mM glucose or 5 mM L-lactate in the media surrounding suspensions of round spermatids (A) or pachytene spermatocytes (B).
As a positive control, cyclopiazonic acid (CPA), which can deplete the ICaS by inhibiting the sarcoplasm-endoplasmic reticulum Ca2+-ATPase (SERCA), was able to induce a rapid increase in [Ca2+]i when added after AA in round spermatids and pachytene spermatocytes under all the conditions tested (with or without external Ca2+ and with lactate or glucose in each of those media; data not shown). These results demonstrate that the rates of [Ca2+]i changes induced by UFAs were not limited by complete depletion of ICaS in the conditions tested in this work.
Pharmacological testing for potential AA metabolism in the AA-induced [Ca2+]i changes in round spermatids
To determine whether AA and its effects on [Ca2+]i could be mediated by cyclooxygenase (COX) or lipoxygenase (LOX) metabolites of AA, inhibitors of COX or LOX were tested (Fig 3). Our results demonstrate that the LOX inhibitor 2-TEDC (5 μM) was able to increase [Ca2+]i on its own (in the absence of external Ca2+). The presence of the COX inhibitor indomethacin (10 μM), but not the LOX inhibitors NDGA (5 μM) or 2-TEDC (5 μM), induced a significant and partial reduction in the kinetics of the changes induced by 4 μM AA (33% decrease, Fig 4). Another AA metabolite, anandamide, was also tested in concentrations ranging from 2–8 μM (Fig 2). This endocanabinoid produced a small change in the rate of [Ca2+]i increase that was only slightly superior to that generated by PA. By contrast, CPA induces a similar rate in the [Ca2+]i change for all experimental groups. Taken together, these results strongly suggest that most of the AA-induced increase of [Ca2+]i was not mediated by eicosanoid or endocannabinoid receptor activation after metabolic conversion of the AA offered to the cells.
Fig 4. Inhibitors of AA metabolism do not affect AA-dependent [Ca2+]i increases.
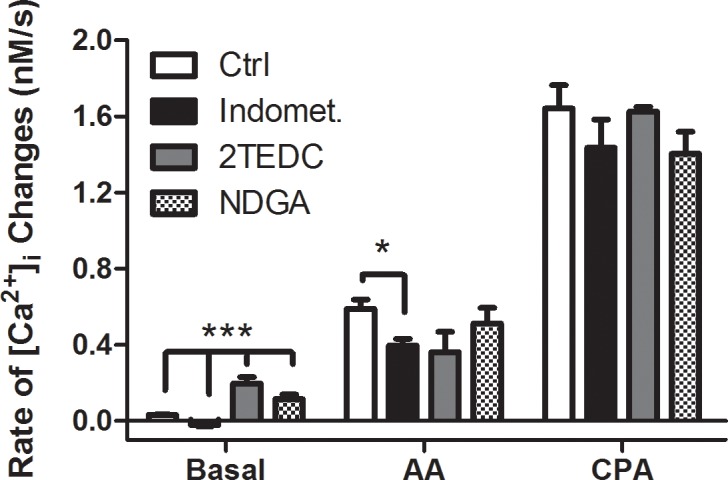
Impact of inhibitors of AA metabolism (COX inhibitor: 10 μM indomethacin, and LOX inhibitors: 5 μM 2TEDC, 5 μM NDGA) on the AA-dependent and 2 μM cyclopiazonic acid dependent increase of [Ca2+]i in KH-lactate media in the absence of external Ca2+. The Mann-Whitney statistical significance test was used to compare rates of [Ca2+]i change with CPA vs. CPA-COX/LOX inhibitors, AA vs. AA-COX/LOX inhibitors and basal rate vs. COX/LOX inhibitors.
Effects of FAs on propidium iodide permeability and LDH release in spermatogenic cells
The entry of propidium iodide (PI) into round spermatids was used to determine whether the FAs in this study affected germ cell integrity (Fig 5). AA was able to induce a significant increase in cell plasma membrane permeability at 16 but not at 8 μM in cells when incubated for more than 30 min (Fig 5A). Interestingly, at 16 μM none of the FAs studied were able to increase plasma membrane permeability in round spermatids after 15 min of incubation. Only 16 μM of DHA induced a significant increase in PI entrance to the cells after 30 min of incubation (Fig 5B). Considering the possibility that spermatids express connexin hemichannels through which PI can permeate [8,43], we next wanted to evaluate LDH leakage as a second criterion of plasma membrane permeability (Fig 5C). The results showed a high degree of variability but were similar to those of PI entry at both time points of FA exposure studied. These results indicate that the changes observed in [Ca2+]i using lower FA concentrations and shorter exposure times (Figs 1–4) were not due to a non-specific FA-induced change in plasma membrane or inner membrane permeabilities.
Fig 5. Effects of FA concentration and exposure time on the membrane integrity of round spermatids.
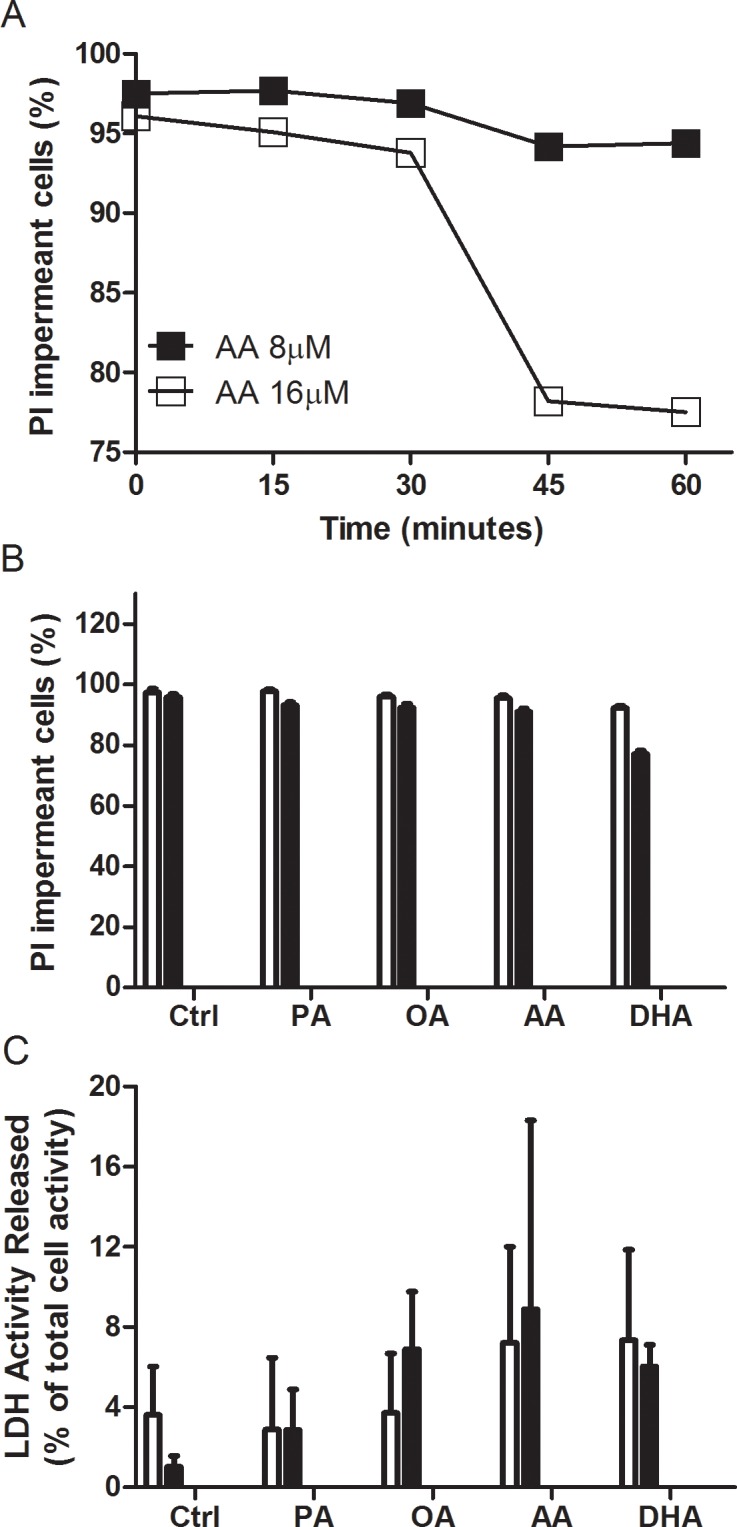
(A) PI nuclear staining was used to monitor membrane integrity over time when 8 or 16 μM AA was applied to round spermatids. (B) PI nuclear staining was used to monitor membrane integrity following induction with 16 μM AA, DHA, OA and PA for 15 min (white bars) and 30 min (black bars) in round spermatids. (C) Percent release of intracellular LDH induced by 16 μM AA, DHA, OA and PA for 15 and 30 min in round spermatids.
Effects of AA on ATP release in spermatogenic cells
Given that unsaturated FAs at > 8 μM concentrations were able to induce entry of PI into spermatogenic cells, and because PI can permeate connexin hemichannels and pannexin channels that play a role in ATP secretion (see [43,44]), we suspected that UFAs could activate these channels. If so, ATP would be released, which, in turn, could activate purinergic signalling in spermatogenic cells [45]. We tested whether ATP was released from spermatogenic cells in response to 15 μM AA. As shown in S1 Fig this was not the case. The ATP liberated from pachytene spermatocytes or round spermatids in response to this relatively high concentration of AA was less than 0.2% of the total ATP content in the cells and below the level of detection in our assay. Addition of 5–100 μM ATP or ADP did not produce significant changes in [Ca2+]i in round spermatids or pachytene spermatocytes (data not shown).
Effects of AA on plasma membrane potential
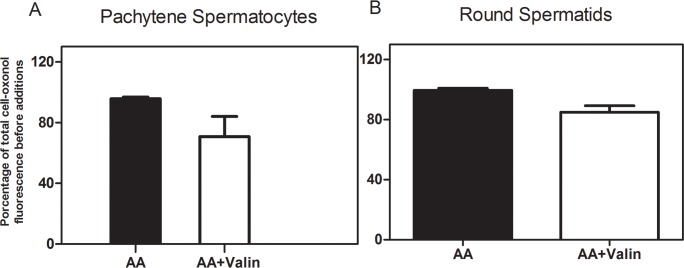
Although our results demonstrated that the changes in [Ca2+]i induced by FA in spermatogenic cells were not associated with entry of Ca2+, the existence of a described voltage-dependence of some GPCRs [46] and voltage-sensing phosphoinositide phosphatases [47] prompted us to look for AA-induced changes in plasma membrane potential. The plasma membrane potential was estimated from the fluorescence of oxonol (Fig 6). An acute (5 min) addition of 4 μM AA did not produce significant changes in the oxonol fluorescence signal in pachytene spermatocytes or round spermatids resuspended in KH buffer, which strongly suggests that at concentrations of 4 μM or less this FA does not modify plasma membrane potential in these cells. As a positive control, valinomycin, a ionophore that increases K+ membrane permeability, induced a significant decrease in oxonol fluorescence when added after AA (Fig 6) in both pachytene spermatocytes and round spermatids, which indicates plasma membrane hyperpolarization [36].
Fig 6. AA does not affect the plasma membrane potential in spermatogenic cells.
Percent change in oxonol fluorescence induced by 4 μM AA with or without 0.5 μM valinomycin in pachytene spermatocytes (A) and round spermatids (B) in KH-lactate media in the absence of external Ca2+.
Effects of AA on intracellular pH
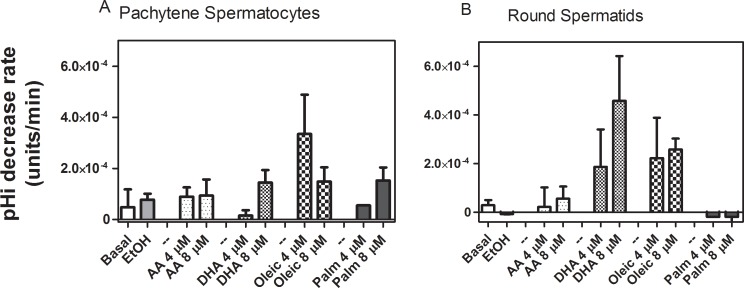
Intracellular alkalinization can increase [Ca2+]i [48] and intracellular acidification can decrease Ca2+ release from ICaS [49,50]. This trend prompted us to examine whether the FA-induced [Ca2+]i changes we observe were really due to pHi changes in spermatogenic cells. As shown in Fig 7, at a concentration of 4 μM none of the UFAs were able to induce a significant change in the pHi of spermatogenic cells.
Fig 7. Effects of FA on pHi changes.
Spermatogenic cells, resuspended in KH media with no Ca2+, were exposed to 4 and 8 μM UFAs or PA. pHi changes were monitored by BCECF fluorescence excitation ratio. Pachytene spermatocytes (A) and round spermatids (B) in KH-lactate media in the absence of external Ca2+.
Effects of FAs on spermatogenic cell mitochondrial membrane potential
Free FAs are known to increase the permeability of mitochondrial inner membranes to H+, thereby inducing a decrease in mitochondrial inner membrane potential and mitochondrial uncoupling [49,51]. Mitochondria can be considered part of ICaS as they provide the ATP for Ca2+-dependent ATPases including SERCA. Therefore, our next question was whether FAs were able to decrease the mitochondrial membrane potential. This parameter was measured by rhodamine 123 fluorescence in permeabilized spermatogenic cells (Fig 8). AA, DHA and OA, but not PA, were able to induce a time-dependent decrease in the mitochondrial membrane potential, which strongly supports the idea that FAs cause a partial uncoupling of the mitochondrial oxidative phosphorylation (Fig 8A). The effects of AA at 1 μM, 2 μM, and 4 μM on the mitochondrial membrane potential were significantly lower in pachytene spermatocytes than in round spermatids (Fig 8B).
Fig 8. UFAs and PA have different effects on the mitochondrial membrane potential of spermatogenic cells.
(A) Comparative effects of different concentrations of AA on mitochondrial membrane potential measured with rhodamine 123 fluorescence in pachytene spermatocytes and round spermatids cells permeabilized with digitonin. (B) Effects of different concentrations of UFAs and PA on round spermatid mitochondrial membrane potential measured with rhodamine 123 fluorescence in cells permeabilized with digitonin.
In spermatids, the slope of the mitochondrial membrane potential vs. FA concentration plot (Fig 8B) indicated that, with the exception of PA, the FAs examined were able to induce a drop in the mitochondrial membrane potential at 1 μM or higher concentrations. AA had the largest effect at all concentrations. Measurements with JC-1 in round spermatids with AA were consistent with these results (data not shown), strongly suggesting a partial mitochondrial uncoupling at 2 μM concentrations or higher.
Effects of AA and DHA on ROS/RNS production in spermatogenic cells
As partial uncoupling of mitochondrial oxidative phosphorylation has been associated with decreased ROS production in this organelle [39,52], we next explored whether the uncoupling caused by UFAs in the mitochondria of round spermatids or pachytene spermatocytes was associated with changes in ROS production in these cells.
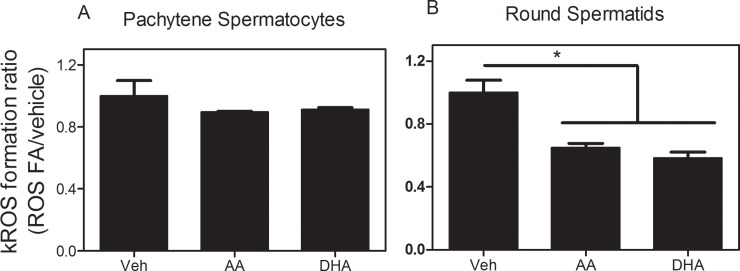
At a concentration of 4 μM, the polyunsaturated FAs AA and DHA did not significantly modify the rate of ROS/RNS production in pachytene spermatocytes (Fig 9A), but induced a considerable decrease in round spermatids (Fig 9B). The cell-type dependent impact of these FAs on ROS/RNS production correlates well with their weaker effects on the mitochondrial membrane potential of spermatocytes relative to spermatids.
Fig 9. Effects of AA and DHA on the relative rate of ROS/RNS production by spermatogenic cells.
At 4 μM of each FA the ROS/RNS levels were measured by the change in the fluorescence of H2DCFDA in pachytene spermatocytes (A) and round spermatids (B).
Caspase 3 activation by AA in pachytene spermatocytes and round spermatids
At concentrations greater than 100 μM, AA can induce in vitro apoptosis in several cell lines [53–55]. Furthermore, [Ca2+]i increase induced by AA has been associated with AA induction of cell apoptosis [56]. In order to determine if similar events were induced by AA in spermatogenic cells, we used caspase 3 activity as a criterion for apoptosis induction both in the absence and presence of external Ca2+.
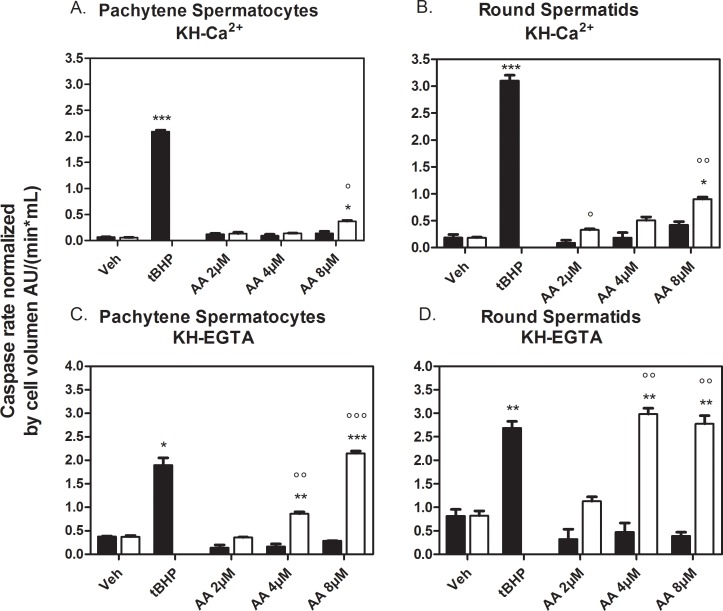
As a positive control, caspase 3 was activated in spermatogenic cells by tBHP with a lag time of approximately 10–20 min after tBHP addition (data not shown). tBHP activated caspase 3 in the absence and presence of external Ca2+ in both cell types. In calcium-free and calcium-containing media, AA-induced caspase 3 activation in spermatocytes and round spermatids but only after 180 min of treatment (Fig 10A and 10B). Interestingly, AA-induced caspase-3 activity at lower concentrations in round spermatids than in pachytene spermatocytes. The absence of external calcium (achieved with EGTA-containing media) caused spermatocytes and spermatids to be more sensitive to AA as it was able to induce caspase-3 at a lower concentration and to a higher extent than in a media with calcium (4 μM vs. 8 μM AA, Fig 9A and 9C). These results suggest that long-term exposures to AA in the seminiferous tubules could induce meiotic and post-meiotic germ cell apoptosis, and that the absence of external Ca2+ can sensitize spermatogenic cells to AA-induced long-term apoptosis.
Fig 10. Effects of AA on caspase 3 activity in spermatogenic cells.
Caspase 3 activity in the presence of external Ca2+ (0.5 mM) (A,B) and no Ca2+-EGTA (C,D) in pachytene spermatocytes (A,C) and round spermatids (B,D), respectively. Caspase 3 activity was measured in arbitrary fluorescence units per min and was normalized to the average cell volumes with diameters of 16 and 11 μm for pachytene spermatocytes and round spermatids, respectively. The final concentration of tBHP was 200 μM. Black bars are the average rates estimated between 0 and 60 min. White bars are the average rates estimated between 180 and 240 min. *Significance with respect to the vehicle. °Significance with respect to the 60 min time point at the same concentration. * or ° = p < 0.05, ** or °° = p < 0.01, *** or °°° = p < 0.001.
Discussion
The regulatory mechanisms that allow SC to control mammalian spermatogenesis are still poorly understood. It is known that SC release a number of soluble factors that could affect proliferation and differentiation of spermatogenic cells [1,57,58]. However, thus far very few of the molecules secreted by SC have been shown to functionally modify spermatogenic cells, especially meiotic and post-meiotic cells (see [59] for insulin-like growth factor II (IGF-I); [60] for activin; [8,9,41,42] for glucose and lactate; [61] for tumour growth factor β (TGFβ). One of the molecules secreted by SC and regulated by FSH is AA [10]. The results of the present study demonstrate that AA, and other UFAs, are able to induce a significant Ca2+ release from ICaS in germ cells. This UFA-dependent [Ca2+]i rise was not mediated by a loss of membrane integrity, by changes in plasma membrane potential, or by activation of ATP release from spermatogenic cells.
Increases in [Ca2+]i were apparently specific to UFAs, as shown by the lack of effect of PA and by the different responses of spermatids to AA and DHA. The effect of OA was intermediate between PA and polyunsaturated FA, as higher concentrations of OA were required to increase [Ca2+]i to levels similar to those induced by AA or DHA. The effects may be ascribed directly to the fatty acids rather than to some of their bioactive metabolites, as shown in the case of AA by the negligible effect of adding anandamide (an AA-derived endo-cannabinoid metabolite) in similar concentrations as AA to the cells, or by pharmacologically blocking the conversion of AA via cyclooxygenase or lipoxygenase inhibitors.
Rhodamine 123 in cells permeabilized with digitonin allowed us to determine the effects of UFAs on the mitochondrial membrane potential. Effects were dependent on cell type and FA species. The ability of UFAs to induce a partial decrease in membrane potential was weaker in pachytene spermatocytes than in round spermatids. In the latter, the magnitude of the UFA-induced decrease in the mitochondrial membrane potential was consistent with what has been described as mild uncoupling [51], and thereby would be expected to decrease the efficiency of mitochondrial ATP synthesis. By measuring intracellular adenine nucleotides, a glucose induced decrease in ATP levels was previously observed to occur in round spermatids to a greater extent than in pachytene spermatocytes due to differences in energy metabolism [42]. As these decreases occurred concomitantly with increases in [Ca2+]i they were ascribed to the ATP-dependent maintenance of Ca2+ homeostasis. This agrees with the results of the present study, in which UFA, in spermatids more so than in spermatocytes, induces a relatively monotonic increase in the rate of Ca2+ release from ICaS and a concomitant decrease in mitochondrial membrane potential as concentrations of UFAs increase.
The different uncoupling effects exerted by UFA on the mitochondria of each of these spermatogenic cell types is possibly linked to the observation that AA and DHA were able to decrease ROS production in round spermatids but not in pachytene spermatocytes. The ability to be partially uncoupled by several stimuli [39] is emerging as an interesting and differential aspect of round spermatid physiology.
The kinetics of AA-induced caspase 3 activation (occurring after 180 min of treatment) demonstrated that this was a late event as compared to AA-induced intracellular Ca2+ release. The fact that caspase 3 activation was more prominent in the absence of external Ca2+ strongly suggests that these cells had shifted to a pro-apoptotic state, likely due to the lowering of ICaS.
Because AA can be released by SC in a FSH-dependent manner [10], and probably other UFAs as well, our results demonstrate that as free FAs, UFAs can modify [Ca2+]i and exhibit different effects on mitochondrial function in pachytene spermatocytes and round spermatids. These results strongly suggest that FAs originating in SC could be part of the cell-cell signalling mechanisms that control or modulate spermatogenic cell functions like metabolism, proliferation, differentiation or death in the seminiferous epithelium.
Supporting Information
(A) Pachytene spermatocytes or (B) round spermatids were exposed to 8 μM AA and subsequently to 25 μg/ml digitonin.
(TIF)
Acknowledgments
We greatly thank Dr. Marta Aveldaño (INIBIBB, Argentina) for her thorough and critical reading of the manuscript and her very helpful editorial work and suggestions. We also thank Ms. Nieves Navarro for the preliminary studies on AA-induced caspase 3 activation in spermatogenic cells.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by Fondo Nacional de Investigación en Ciencia Y Tecnologia (Fondecyt) grant numbers 1110267 and 1140758 to JGR, and by the Pontificia Universidad Catolica de Valparaiso (PUCV-VRIEA) grant 125.726 to JGR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jegou B. The Sertoli-germ cell communication network in mammals. Int Rev Cytol. 1993;147: 25–96 [PubMed] [Google Scholar]
- 2.Turner TT, D'Addario DA, Howards SS. The transepithelial movement of 3H-3-O-methyl-D-glucose in the hamster seminiferous and cauda epididymidal tubules. Fertil Steril. 1983;40: 530–535 [DOI] [PubMed] [Google Scholar]
- 3.Robinson R, Fritz IB. Metabolism of glucose by Sertoli cells in culture. Biol Reprod. 1981;24: 1032–1041 [DOI] [PubMed] [Google Scholar]
- 4.Mita M, Price JM, Hall PF. Stimulation by follicle-stimulating hormone of synthesis of lactate by Sertoli cells from rat testis. Endocrinology. 1982;110: 1535–1541 [DOI] [PubMed] [Google Scholar]
- 5.Le GF, Attramadal H, Borrebaek B, Horn R, Froysa A, Tvermyr M, et al. Effects of FSH, isoproterenol, and cyclic AMP on the production of lactate and pyruvate by cultured Sertoli cells. Arch Androl. 1983;10: 149–154 [DOI] [PubMed] [Google Scholar]
- 6.Hall PF, Mita M. Influence of follicle-stimulating hormone on glucose transport by cultured Sertoli cells. Biol Reprod. 1984;31: 863–869 [DOI] [PubMed] [Google Scholar]
- 7.Riera MF, Meroni SB, Gomez GE, Schteingart HF, Pellizzari EH, Cigorraga SB. Regulation of lactate production by FSH, iL1beta, and TNFalpha in rat Sertoli cells. Gen Comp Endocrinol. 2001;122: 88–97 [DOI] [PubMed] [Google Scholar]
- 8.Reyes JG, Herrera E, Lobos L, Salas K, Lagos N, Jorquera RA, et al. Dynamics of intracellular calcium induced by lactate and glucose in rat pachytene spermatocytes and round spermatids. Reproduction. 2002;123: 701–710 [DOI] [PubMed] [Google Scholar]
- 9.Bustamante-Marin X, Quiroga C, Lavandero S, Reyes JG, Moreno RD. Apoptosis, necrosis and autophagy are influenced by metabolic energy sources in cultured rat spermatocytes. Apoptosis. 2012;17: 539–550 10.1007/s10495-012-0709-2 [DOI] [PubMed] [Google Scholar]
- 10.Jannini EA, Ulisse S, Cecconi S, Cironi L, Colonna R, D'Armiento M, et al. Follicle-stimulating hormone-induced phospholipase A2 activity and eicosanoid generation in rat Sertoli cells. Biol Reprod. 1994;51: 140–145 [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138: 2081–2088 [DOI] [PubMed] [Google Scholar]
- 12.Lizama C, Alfaro I, Reyes JG, Moreno RD. Up-regulation of CD95 (Apo-1/Fas) is associated with spermatocyte apoptosis during the first round of spermatogenesis in the rat. Apoptosis. 2007;12: 499–512 [DOI] [PubMed] [Google Scholar]
- 13.Ulisse S, Cinque B, Silvano G, Rucci N, Biordi L, Cifone MGet al. Erk-dependent cytosolic phospholipase A2 activity is induced by CD95 ligand cross-linking in the mouse derived Sertoli cell line TM4 and is required to trigger apoptosis in CD95 bearing cells. Cell Death Differ. 2000;7: 916–924 [DOI] [PubMed] [Google Scholar]
- 14.Meroni SB, Riera MF, Pellizzari EH, Schteingart HF, Cigorraga SB. Possible role of arachidonic acid in the regulation of lactate production in rat Sertoli cells. Int J Androl. 2003;26: 310–317 [DOI] [PubMed] [Google Scholar]
- 15.MacDonald ML, Rogers QR, Morris JG, Cupps PT. Effects of linoleate and arachidonate deficiencies on reproduction and spermatogenesis in the cat. J Nutr. 1984;114: 719–726 [DOI] [PubMed] [Google Scholar]
- 16.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, et al. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J Biol Chem. 2004;279: 38194–38200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrillo C, Del Mar CM, Roelofs H, Wanten G, Alonso-Torre SR. Activation of human neutrophils by oleic acid involves the production of reactive oxygen species and a rise in cytosolic calcium concentration: a comparison with N-6 polyunsaturated fatty acids. Cell Physiol Biochem. 2011;28: 329–338 10.1159/000331749 [DOI] [PubMed] [Google Scholar]
- 18.Yeung-Yam-Wah V, Lee AK, Tse A. Arachidonic acid mobilizes Ca2+ from the endoplasmic reticulum and an acidic store in rat pancreatic beta cells. Cell Calcium. 2012;51: 140–148 10.1016/j.ceca.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 19.Chiarella P, Puglisi R, Sorrentino V, Boitani C, Stefanini M. Ryanodine receptors are expressed and functionally active in mouse spermatogenic cells and their inhibition interferes with spermatogonial differentiation. J Cell Sci. 2004;117: 4127–4134 [DOI] [PubMed] [Google Scholar]
- 20.Mishra DP, Pal R, Shaha C. Changes in cytosolic Ca2+ levels regulate Bcl-xS and Bcl-xL expression in spermatogenic cells during apoptotic death. J Biol Chem. 2006;281: 2133–2143 [DOI] [PubMed] [Google Scholar]
- 21.Yuan W, Leisner TM, McFadden AW, Clark S, Hiller S, Maeda N, et al. CIB1 is essential for mouse spermatogenesis. Mol Cell Biol. 2006;26: 8507–8514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Kim H, Kim DH, Gye MC. Effects of calcium channel blockers on the spermatogenesis and gene expression in peripubertal mouse testis. Arch Androl. 2006;52: 311–318 [DOI] [PubMed] [Google Scholar]
- 23.Wu JY, Ribar TJ, Cummings DE, Burton KA, McKnight GS, Means AR. Spermiogenesis and exchange of basic nuclear proteins are impaired in male germ cells lacking Camk4. Nat Genet. 2000;25: 448–452 [DOI] [PubMed] [Google Scholar]
- 24.Oresti GM, Reyes JG, Luquez JM, Osses N, Furland NE, Aveldaño MI. Differentiation-related changes in lipid classes with long-chain and very long-chain polyenoic fatty acids in rat spermatogenic cells. J Lipid Res. 2010;51: 2909–2921 10.1194/jlr.M006429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roqueta-Rivera M, Stroud CK, Haschek WM, Akare SJ, Segre M, Brush RS, et al. Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J Lipid Res. 2010;51: 360–367 10.1194/jlr.M001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lappin DF, Whaley K. Prostaglandins and prostaglandin synthetase inhibitors regulate the synthesis of complement components by human monocytes. Clin Exp Immunol. 1982;49: 623–630 [PMC free article] [PubMed] [Google Scholar]
- 27.Goetzl EJ. A role for endogenous mono-hydroxy-eicosatetraenoic acids (HETEs) in the regulation of human neutrophil migration. Immunology. 1980;40: 709–719 [PMC free article] [PubMed] [Google Scholar]
- 28.Cho H, Ueda M, Tamaoka M, Hamaguchi M, Aisaka K, Kiso Y, et al. Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J Med Chem. 1991;34: 1503–1505 [DOI] [PubMed] [Google Scholar]
- 29.Romrell LJ, Bellve AR, Fawcett DW. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol. 1976;49: 119–131 [DOI] [PubMed] [Google Scholar]
- 30.Reyes JG, Diaz A, Osses N, Opazo C, Benos DJ. On stage single cell identification of rat spermatogenic cells. Biol Cell. 1997;89: 53–66 [DOI] [PubMed] [Google Scholar]
- 31.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260: 3440–3450 [PubMed] [Google Scholar]
- 32.Emaus RK, Grunwald R, Lemasters JJ. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986;850: 436–448 [DOI] [PubMed] [Google Scholar]
- 33.Scaduto RC Jr., Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76: 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyes J, Benos DJ. Changes in interfacial potentials induced by carbonylcyanide phenylhydrazone uncouplers: possible role in inhibition of mitochondrial oxygen consumption and other transport processes. Membr Biochem. 1984;5: 243–268 [DOI] [PubMed] [Google Scholar]
- 35.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991;88: 3671–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reyes JG, Bacigalupo J, Araya R, Benos DJ. Ion dependence of resting membrane potential of rat spermatids. J Reprod Fertil. 1994;102: 313–319 [DOI] [PubMed] [Google Scholar]
- 37.Rink TJ, Tsien RY, Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982;95: 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyman GE, DeVincenzo JP. Determination of picogram amounts of ATP using the luciferin-luciferase enzyme system. Anal Biochem. 1967;21: 435–443 [DOI] [PubMed] [Google Scholar]
- 39.Pino JA, Osses N, Oyarzun D, Farias JG, Moreno RD, Reyes JG. Differential effects of temperature on reactive oxygen/nitrogen species production in rat pachytene spermatocytes and round spermatids. Reproduction. 2013;145: 203–212 10.1530/REP-12-0330 [DOI] [PubMed] [Google Scholar]
- 40.Sokal RR, Rohlf FJ. Biometría, principios y métodos estadísticos en la investigación biológica1979;
- 41.Reyes JG, Osses N, Knox M, Darszon A, Trevino CL. Glucose and lactate regulate maitotoxin-activated Ca2+ entry in spermatogenic cells: the role of intracellular [Ca2+]. FEBS Lett. 2010;584: 3111–3115 10.1016/j.febslet.2010.05.051 [DOI] [PubMed] [Google Scholar]
- 42.Herrera E, Salas K, Lagos N, Benos DJ, Reyes JG. Energy metabolism and its linkage to intracellular Ca2+ and pH regulation in rat spermatogenic cells. Biol Cell. 2000;92: 429–440 [DOI] [PubMed] [Google Scholar]
- 43.Pointis G, Gilleron J, Carette D, Segretain D. Testicular connexin 43, a precocious molecular target for the effect of environmental toxicants on male fertility. Spermatogenesis. 2011;1: 303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turmel P, Dufresne J, Hermo L, Smith CE, Penuela S, Laird DW, et al. Characterization of pannexin1 and pannexin3 and their regulation by androgens in the male reproductive tract of the adult rat. Mol Reprod Dev. 2011;78: 124–138 10.1002/mrd.21280 [DOI] [PubMed] [Google Scholar]
- 45.Glass R, Bardini M, Robson T, Burnstock G. Expression of nucleotide P2X receptor subtypes during spermatogenesis in the adult rat testis. Cells Tissues Organs. 2001;169: 377–387 [DOI] [PubMed] [Google Scholar]
- 46.Mahaut-Smith MP, Martinez-Pinna J, Gurung IS. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci. 2008;29: 421–429 10.1016/j.tips.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 47.Okamura Y, Murata Y, Iwasaki H. Voltage-sensing phosphatase: actions and potentials. J Physiol. 2009;587: 513–520 10.1113/jphysiol.2008.163097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, Hao B, Lu Y, Yu P, Lee HC, Yue J. Intracellular alkalinization induces cytosolic Ca2+ increases by inhibiting sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). PLoS One. 2012;7: e31905– 10.1371/journal.pone.0031905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rousseau E, Pinkos J. pH modulates conducting and gating behaviour of single calcium release channels. Pflugers Arch. 1990;415: 645–647 [DOI] [PubMed] [Google Scholar]
- 50.Balnave CD, Vaughan-Jones RD. Effect of intracellular pH on spontaneous Ca2+ sparks in rat ventricular myocytes. J Physiol. 2000;528 Pt 1: 25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di PM, Lorusso M. Interaction of free fatty acids with mitochondria: coupling, uncoupling and permeability transition. Biochim Biophys Acta. 2006;1757: 1330–1337 [DOI] [PubMed] [Google Scholar]
- 52.Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011;51: 1106–1115 10.1016/j.freeradbiomed.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 53.Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A. 1998;95: 681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha aopototic signaling. J Biol Chem. 2001;276: 12035–12040 [DOI] [PubMed] [Google Scholar]
- 55.Artwohl M, Lindenmair A, Roden M, Waldhausl WK, Freudenthaler A, Klosner G, et al. Fatty acids induce apoptosis in human smooth muscle cells depending on chain length, saturation, and duration of exposure. Atherosclerosis. 2009;202: 351–362 10.1016/j.atherosclerosis.2008.05.030 [DOI] [PubMed] [Google Scholar]
- 56.Liu WH, Chang LS. Arachidonic acid induces Fas and FasL upregulation in human leukemia U937 cells via Ca2+/ROS-mediated suppression of ERK/c-Fos pathway and activation of p38 MAPK/ATF-2 pathway. Toxicol Lett. 2009;191: 140–148 10.1016/j.toxlet.2009.08.016 [DOI] [PubMed] [Google Scholar]
- 57.Huleihel M, Abuelhija M, Lunenfeld E. In vitro culture of testicular germ cells: regulatory factors and limitations. Growth Factors. 2007;25: 236–252 [DOI] [PubMed] [Google Scholar]
- 58.Sofikitis N, Giotitsas N, Tsounapi P, Baltogiannis D, Giannakis D, Pardalidis N. Hormonal regulation of spermatogenesis and spermiogenesis. J Steroid Biochem Mol Biol. 2008;109: 323–330 10.1016/j.jsbmb.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 59.Tsuruta JK O'Brien DA. Sertoli cell-spermatogenic cell interaction: the insulin-like growth factor-II/cation-independent mannose 6-phosphate receptor mediates changes in spermatogenic cell gene expression in mice. Biol Reprod. 1995;53: 1454–1464 [DOI] [PubMed] [Google Scholar]
- 60.Meinhardt A, McFarlane JR, Seitz J, de Kretser DM. Activin maintains the condensed type of mitochondria in germ cells. Mol Cell Endocrinol. 2000;168: 111–117 [DOI] [PubMed] [Google Scholar]
- 61.Konrad L, Keilani MM, Laible L, Nottelmann U, Hofmann R. Effects of TGF-betas and a specific antagonist on apoptosis of immature rat male germ cells in vitro. Apoptosis. 2006;11: 739–748 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Pachytene spermatocytes or (B) round spermatids were exposed to 8 μM AA and subsequently to 25 μg/ml digitonin.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.