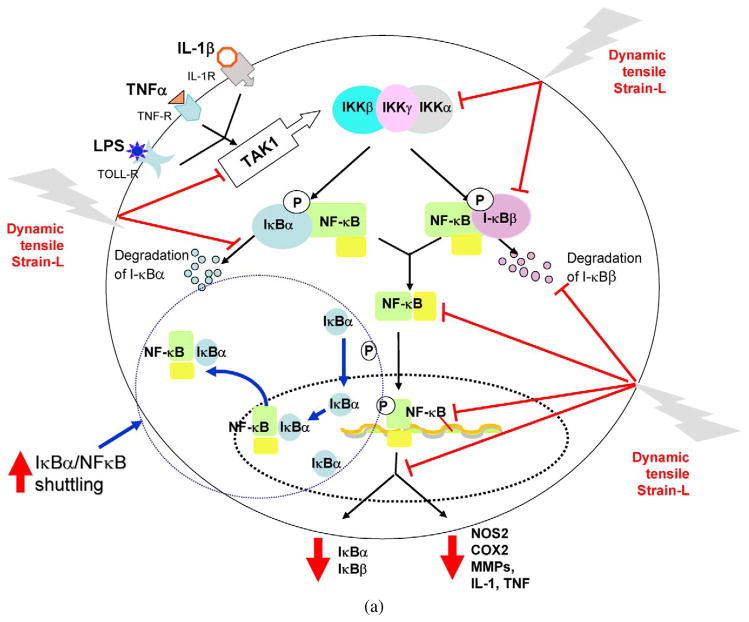
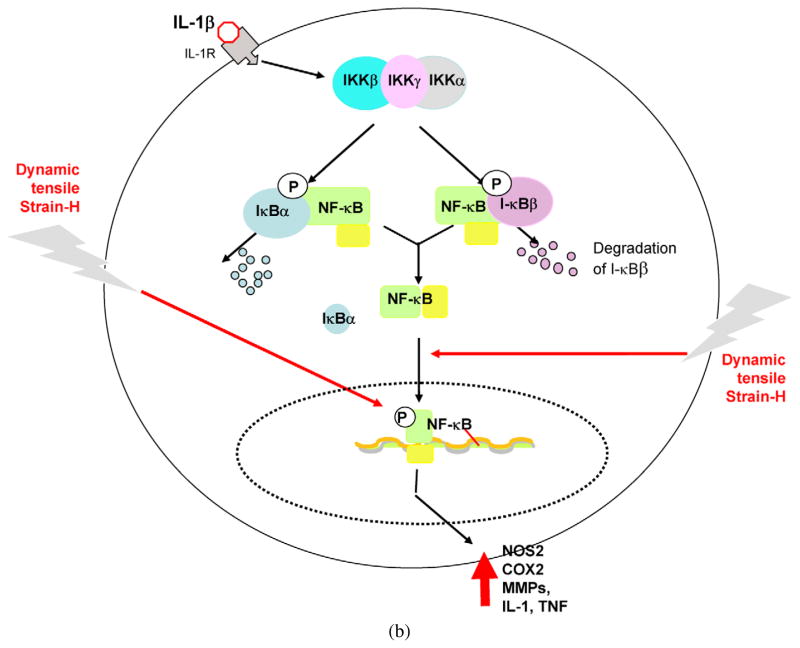
Fig. 3.
Schematic respresentation of the mechanisms of intracellular actions of dynamic tensile strain. (a) Cyclic/dynamic tensile strain of low magnitudes (CTS-L) suppresses IL-1β induced proinflammatory gene induction by intercepting salient steps in the NF-κB signaling cascade to inhibit its transcriptional activity. (i) CTS inhibits TAK1 activation, the point of convergence of signals generated by IL-1β, TNF-α or LPS, to subsequently inhibit IKK. Inhibition of IKK activation leads to suppression of phosphorylation and degradation of I-κBα and I-κBβ. This is followed by the failure of NF-κB dissociation from I-κBα and I-κBβ and thus inhibition of its nuclear translocation. (ii) At the initial stages of IL-1β-mediated activation of cells, CTS-L upregulates I-κBα nuclear translocation to prevent NF-κB binding to the DNA and facilitate export of nuclear NF-κB, that may enter the nucleus. (iii) CTS also suppresses IL-1β-induced I-κBα and I-κBβ mRNA expression. These actions collectively inhibit proinflammatory gene induction as well as expression of molecules involved in NF-κB signaling cascade to suppress inflammation. Thin arrows indicate NF-κB signaling pathway activated by IL-1β. The stop arrows indicate the points where CTS-L intercepts the NF-κB signaling cascade. The arrows in I-κBα/NF-κB shuttling circle show the mechanisms by which I-κBα shuttles the NF-κB out of the nucleus. (b) Cyclic/dynamic tensile strain of high magnitudes (DTS-H) upregulates proinflammatory gene transcription by inducing I-κBα and I-κBβ degradation and subsequent nuclear translocation of NF-κB. This results in the transcriptional activation of proinflammatory mediators including NOS2, COX-2, MMPs, IL-1β and TNF-α, and inhibition of the expression of matrix associate proteins, aggrecan, collagen type II and TIMPs. Thin arrows indicate pathway regulated by IL-1β. Heavy arrows indicate points where CTS-H is as yet known to activate NF-κB signaling cascade.