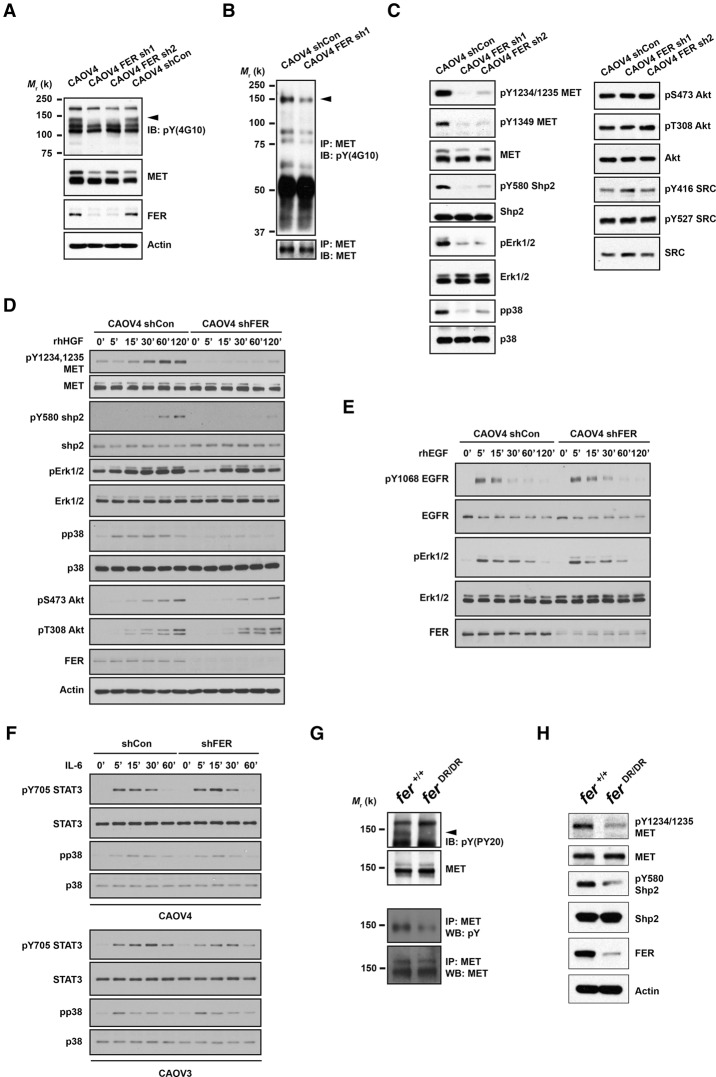
Figure 2.
Loss of FER attenuated tyrosine phosphorylation of HGFR (MET) and MET-mediated downstream signaling. (A) Anti-phosphotyrosine antibody immunoblot (4G10) to examine the impact of FER knockdown on tyrosine phosphorylation in CAOV4 cells. The blot was reprobed with antibodies against MET, FER, and the loading control actin. (B) Tyrosine phosphorylation of MET was examined by immunoprecipitation followed by blotting with anti-phosphotyrosine antibody 4G10. The blot was reprobed for MET to indicate equal immunoprecipitation efficiency and loading. (C) CAOV4 cells expressing either control or FER shRNA were lysed and immunoblotted as indicated to measure the activation of MET and MET-regulated downstream signaling pathways. (D–F) Cells were serum-starved and stimulated with recombinant human HGF (D), EGF (E), or IL-6 (F) for the indicated times; lysed; and immunoblotted with the designated antibodies to illustrate the impact of FER deficiency on HGF-, EGF-, or IL-6-induced signaling. (G, top) Anti-phosphotyrosine antibody immunoblot (PY20) to examine the effects of a destabilized FER-D743R mutant on tyrosine phosphorylation in mouse embryonic fibroblasts (MEFs). The position of MET is indicated by an arrowhead. The blot was reprobed for MET. (Bottom) MET was immunoprecipitated from both Fer+/+ and FerDR/DR MEF cell lysates, and tyrosine phosphorylation was examined by immunoblotting with antibody 4G10. (H) Fer+/+ and FerDR/DR MEF cells were lysed and immunoblotted as indicated to illustrate the impact of FER destabilization on MET and MET-mediated downstream signaling pathways.