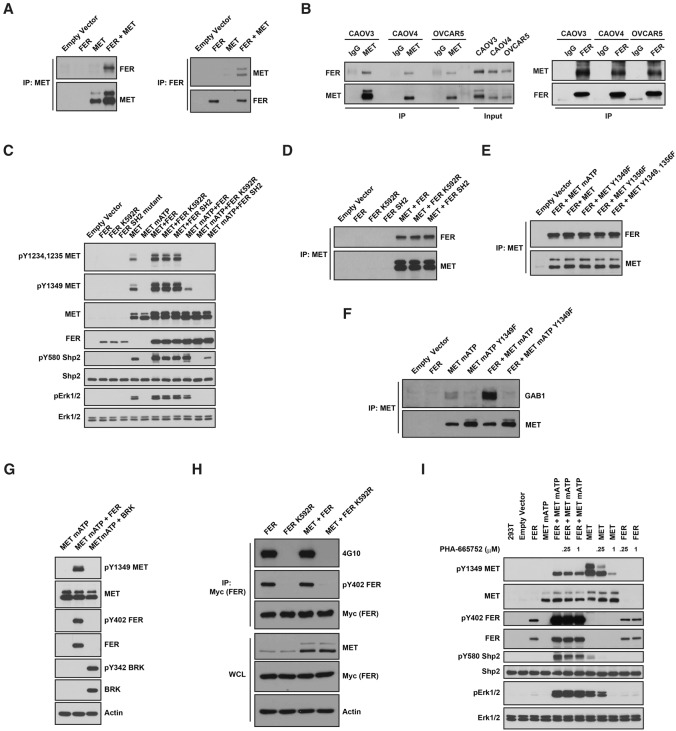
Figure 4.
FER bound to and phosphorylated MET at Tyr1349. (A) After transient transfection in 293T cells, MET was immunoprecipitated and probed for association with FER (left) and vice versa (right). (B) Endogenous MET was immunoprecipitated from CAOV4, CAOV3, and OVCAR5 ovarian cancer cells and probed for association with FER (left) and vice versa (right). (C) 293T cells were transiently transfected with the indicated constructs, lysed, and immunoblotted as indicated to illustrate the phosphorylation of MET by FER and its impact on downstream SHP2–ERK signaling. (D) MET was coexpressed with either wild-type or mutant forms of FER (kinase-dead K592R or SH2 mutant) in 293T cells to compare the effects of mutations in FER on its association with MET. (E) FER was coexpressed with either wild-type or Tyr→Phe mutants of MET (Y1349F, Y1356F, or YY1349,1356FF) in 293T cells to compare the effects of the MET point mutations on its association with FER. (F) FER was expressed alone or with either mATP or mATP Y1349F mutant forms of MET in 293T cells. MET was immunoprecipitated, and the association with GAB1 in each sample was assessed by immunoblotting. (G) 293T cells were transiently transfected with MET mATP alone or together with either FER or BRK, and the phosphorylation of MET on Tyr1349 was assessed by immunoblotting. (H) 6xMYC-tagged wild-type or inactive K592R FER was expressed alone or cotransfected with MET, immunoprecipitated with anti-MYC antibody, and probed for tyrosine phosphorylation with either 4G10 (global Tyr phosphorylation) or pTyr402-FER-specific antibodies. (I) Transfected 293T cells were treated with or without MET inhibitor PHA-665752 (4 h), as indicated, and the cell lysates were immunoblotted with the designated antibodies to illustrate the effect of the small molecule inhibitor on MET phosphorylation and downstream SHP2–ERK signaling.