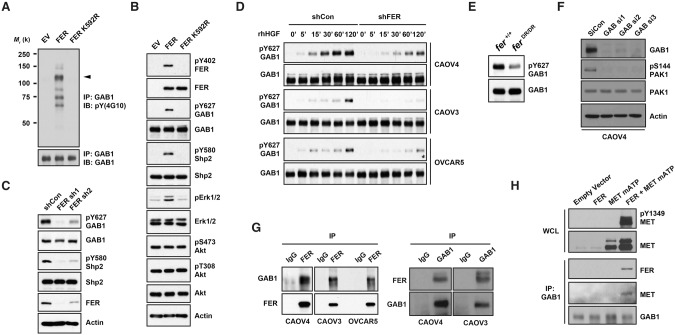
Figure 5.
FER formed a complex with GAB1 via MET and phosphorylated GAB1 at Tyr627. 293T cells were transiently transfected with either wild-type FER or a kinase-dead FER-K592R mutant. (A) Tyrosine phosphorylation of GAB1 was examined by immunoprecipitation and blotting with anti-phosphotyrosine antibody 4G10. (B) The whole-cell lysates were immunoblotted as indicated to show the increased tyrosine phosphorylation of GAB1 and SHP2 and the impact on downstream signaling. (C) Tyrosine phosphorylation of GAB1 and SHP2 was compared in CAOV4 cells expressing either control or FER targeted shRNAs. (D) Cells were serum-starved and stimulated with hHGF for the indicated times, lysed, and immunoblotted with both pTyr627 and total GAB1 antibodies to demonstrate FER-regulated GAB1 phosphorylation. (E) Phosphorylation status of Tyr627 of GAB1 in Fer+/+ and FerDR/DR MEF cells. (F) Control or GAB1 siRNAs were delivered into CAOV4 cells by electroporation. After 48 h, the expression levels of GAB1 and activation of PAK1 were measured by immunoblotting. (G) Endogenous FER was immunoprecipitated from CAOV4, CAOV3, and OVCAR5 ovarian cancer cells, and its association with GAB1 was examined by immunoblotting (left) and vice versa (right). (H) Endogenous GAB1 was immunoprecipitated from lysates of 293T cells transfected with FER and the MET mATP mutant alone or together. The association of FER and MET was assessed by immunoblotting.