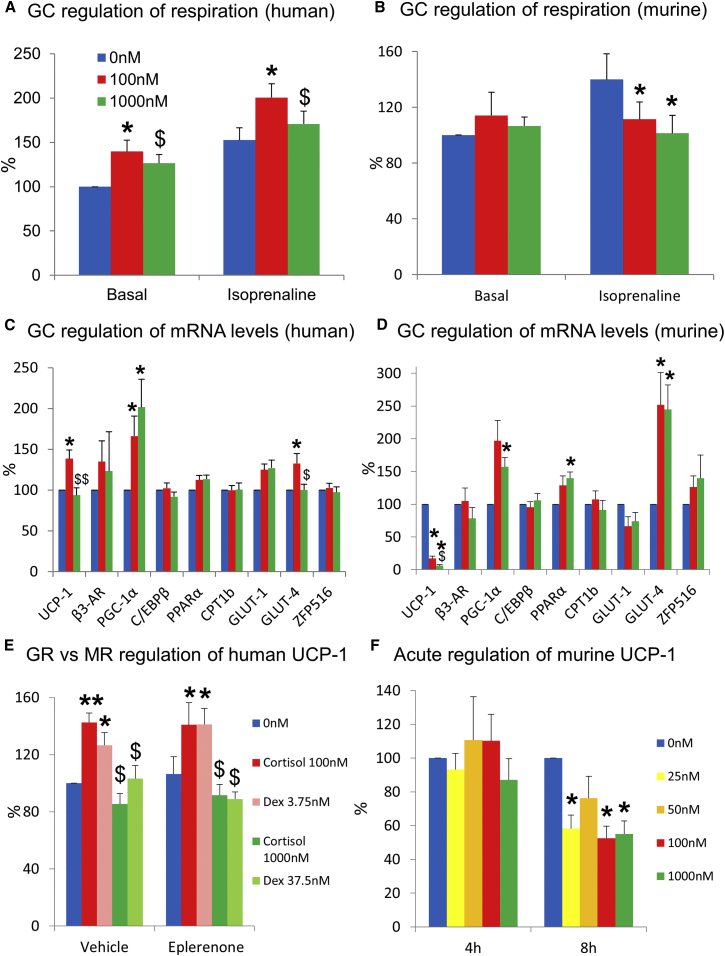
Figure 5.
Glucocorticoid Regulation of Human and Murine Brown Adipocytes In Vitro
(A and B) Data are mean ± SEM for paired (A) human brown adipocytes (n = 6) or (B) murine inguinal adipocytes (n = 7) cultured for 24 hr in either 0 (blue columns), 100 (red columns), or 1,000 nM (green columns) cortisol. Cortisol (100 nM) increased basal and isoprenaline-stimulated oxygen consumption compared with 0 and 1,000 nM in the human brown adipocytes, but 100 and 1,000 nM cortisol decreased isoprenaline-stimulated oxygen consumption in the murine adipocytes, with basal 0 nM normalized to 100%.
(C and D) mRNA levels (with 0 nM normalized to 100%) from paired (C) human brown adipocytes (n = 8) or (D) inguinal beige adipocytes (n = 6) following 24 hr incubation with cortisol at 0, 100, and 1,000 nM. Cortisol (100 nM) increased UCP-1 levels in human brown adipocytes but decreased UCP-1 in murine adipocytes.
(E) UCP-1 mRNA levels from paired primary human brown adipocytes (n = 7) following 24 hr incubation with cortisol at 0, 100, and 1,000 nM or equivalent concentrations (3.75 [pale red columns] and 37.5 nM [pale green columns]) of the selective glucocorticoid (GC) receptor (GR) agonist dexamethasone (Dex) ± the mineralocorticoid receptor (MR) antagonist eplerenone (10 μM). Low-dose cortisol and dexamethasone increased UCP-1 mRNA levels, while eplerenone did not alter UCP-1.
(F) UCP-1 mRNA levels from paired interscapular murine brown adipocytes (n = 6) following 4 and 8 hr incubation with 0, 25 (yellow columns), 50 (orange columns), 100, and 1,000 nM cortisol. Low- and high-dose cortisol suppressed UCP-1 following 8 hr incubation. Data were analyzed by repeated-measures ANOVA with post hoc Bonferroni testing. ∗p < 0.05, ∗∗p < 0.01 versus 0 nM; $p < 0.05, $$p < 0.01 versus 100 nM cortisol.