Abstract
Irisin is processed from fibronectin type III domain-containing protein 5 (FNDC5). However, a controversy exists concerning irisin origin, regulation and function. To elucidate the relationship between serum irisin and FNDC5 mRNA expression levels, we evaluated plasma irisin levels and FNDC5 gene expression in the hypothalamus, gastrocnemius muscle and different depots of adipose tissue in models of altered metabolism. In normal rats, blood irisin levels diminished after 48-h fast and with leptin, insulin and alloxan treatments, and serum irisin concentrations increased in diabetic rats after insulin treatment and acute treatments of irisin increased blood insulin levels. No changes were observed during long-term experiments with different diets. We suggested that levels of circulating irisin are the result of the sum of the irisin produced by different depots of adipose tissue and skeletal muscle. This study shows for the first time that there are differences in FNDC5 expression depending on white adipose tissue depots. Moreover, a considerable decrease in visceral and epididymal adipose tissue depots correlated with increased FNDC5 mRNA expression levels, probably in an attempt to compensate the decrease that occurs in their mass. Hypothalamic FNDC5 expression did not change for any of the tested diets but increased with leptin, insulin and metformin treatments suggesting that the regulation of central and peripheral FNDC5/irisin expression and functions are different.
Metabolic syndrome is characterized by a series of disorders such as, obesity, insulin resistance, glucose intolerance and hyperlipidemia. Obesity is a driver of metabolic syndrome and has emerged as one of the most important medical problems of the 21st century that is a major risk factor for the development of cardiovascular diseases and type 2 diabetes (T2D). According to a World Health Organization estimate, diabetes mellitus affects 346 million people worldwide1 and its prevalence continues to increase due to advancing age, increasing obesity rates and inactivity2,3.
Obesity is a very complex phenomenon in which plays very important roles peripheral tissues such as adipose tissue, liver and muscle as well the neurohormonal and neurotransmitter dysregulation4,5,6,7,8. In this regard, the hypothalamus plays a key role in energy homeostasis. It controls food intake and senses multiple circulating peripheral signals such as leptin, insulin, adiponectin, gut hormones and nutrients that function as “indicators” that interact with specific hypothalamic regions to disturb the energy balance both peripherally and centrally9,10,11.
In 2012, Boström and colleagues discovered that in muscle, exercise increases the expression of FNDC5 (Fibronectin type III domain-containing protein 5), a membrane protein encoded by the FNDC5 gene. The FNDC5 protein is cleaved and secreted as a new hormone called irisin12, suggesting that some of beneficial effects of exercise could be mediated by this hormone. Irisin induces the browning of white adipose tissue (WAT), thereby increasing thermogenesis and possibly improving glucose homeostasis12,13,14. There have been many studies attempting to correlate plasma irisin levels with metabolic disorders such as obesity, diabetes, non-alcoholic fatty liver disease (NAFLD) and polycystic ovary syndrome (PCOS) (both associated with metabolic syndrome), however, the results were not consistent among the various studies (reviewed by15). Recently, it has been reported that irisin is also produced by adipocytes14,16 and that it is present in human cerebrospinal fluid (CSF), although its role in the brain is largely unknown. FNDC5 inhibition reduces neurogenesis17, while its overexpression stimulates neural differentiation18, and pharmacological doses of irisin increase the proliferation of mouse hippocampal neuronal cells19 similar to the effects of endurance exercise, a process associated with the increased expression of brain derived neurotrophic factor (BDNF)20. In the hypothalamus, irisin appears colocalized with neuropeptide Y (NPY) in the neuronal cells of the paraventricular nucleus21. These findings support the idea that irisin has both peripheral and central functions. Despite the promising results, many aspects of the regulation, secretion and physiological aspects of irisin remain unknown. Some previous studies even questioned the existence of irisin22, although that point was clarified recently by Jedrychowski and coworkers23 who showed that human irisin exists, circulates, and is regulated by exercise.
The main aim of this study was to test the hypothesis that metabolic status regulates both central and peripheral FNDC5 mRNA expression levels and irisin synthesis and secretion. Most of the published works studied the effects of irisin on browning in subcutaneous adipose tissue, but few did so on other fatty deposits16. To elucidate the relationship between serum irisin and FNDC5 mRNA expression levels, we evaluated FNDC5 mRNA expression levels in the hypothalamus, gastrocnemius muscle, brown adipose tissue and visceral (abdominal), epididymal and subcutaneous adipose tissue. We used different models of altered metabolism: rats reared with a high-fat diet (HFD) or caloric restriction (CR) for three months, fasting, and treatments with leptin, insulin, metformin, alloxan and alloxan plus insulin.
Materials and Methods
Animals
All experiments and animal protocols involved in this study were reviewed and approved by the Ethics Committees of the University of A Coruña and CHUAC, in accordance with EU Normative for the use of experimental animals. Wistar rats of different ages used in this study were housed in a temperature controlled room with a 12 h light, 12 h dark cycle (lights from 08:00 to 20:00 h). To study the expression of profile of FNDC5 mRNAs we used females to obtain ovaries and placenta samples in the remaining experiments we used males. All rats were provided with ad libitum access to water.
Body composition and tissue dissection
Liver and visceral and epididymal WAT were dissected and weighed, and somatic indices were calculated as the ratio between tissue and body weights. Rats were killed by cervical dislocation and trunk blood was extracted. The hypothalami were dissected and stored at −80 °C until further processing for real-time PCR assays. The hypothalamus was defined by the posterior margin of the optic chiasm and the anterior margin of the mammillary bodies to a depth of approximately 2 mm, following previous protocols24.
Quantitative real-time PCR
Total RNA was extracted from tissues using TRIzol reagent (Invitrogen). RNA quality and concentrations were determined by agarose gel electrophoresis and spectrophotometry in a ND-1000 NANODROP 385 spectrophotometer (Thermo-Scientific), respectively. Real-time PCR was performed on a Roche LightCycler 480 Real Time PCR Detection System. For mRNA quantification, 1 μg of total RNA per tissue sample was treated with RQ1 RNAse-free DNAse-I (Promega) and retro-transcribed (RT) in a 30 μl reaction, using M-MLV reverse transcriptase and random primers (Invitrogen). For PCR, we used SYBR Green qPCR Master Mix (Roche). The primers used were: HPRT forward 5′-AGCCGACCGGTTCTGTCAT-3′; HPRT reverse 5′-GGTCAATAACCTGGTTCATCATCAC-3′; FNDC5 forward 5′-GAGGTGCTGATCATCGTCGT-3′; FNDC5 reverse 5′-GAGCAAGCACTGAAAGGGTTT-3′; SOCS3 forward 5′-ACCACTACATGCCGCCCCCA-3′; SOCS3 reverse 5′-TCGGCTCAGTACCAGCGGGA-3′; UCP1 forward 5′-CAATGACCATGTACACCAAGGAA-3′; UCP1 reverse 5′-GATCCGAGTCGCAGAAAAGAA-3′. For data analysis, the input value of the target gene was standardized to the HPRT value for each sample. PCR was initiated by one hold of 95 °C for 10 min, followed by 40 cycles of 15 s at 95 °C, 55 s at 60 °C, and 5 s at 72 °C, followed by one hold of 72 °C for 10 min.
Plasma measurements
Irisin and insulin levels were measured in plasma samples by commercially available ELISA kits according to the manufacturer’s specifications (Adipogen and DRG respectively). Intra- and inter- assay coefficients of variations for ELISAs were below 8% and 10% (irisin) and 3% and 5% (insulin) respectively.
Experimental design
Tissue distribution and differential expression of the FNDC5 gene in rats
The expression of profile of FNDC5 mRNAs was explored in a broad panel of tissues from adult (>75 days-old, n = 3) male and female rats. The tissues were removed, quick frozen and stored at −80 °C until used for RT-PCR expression analyses.
Alterations in FNDC5 gene expression and circulating irisin levels in rats reared with different diets: Long-term experiments
In order to define the hypothalamic, skeletal muscle and brown and white adipose tissue FNDC5 mRNA profiles and serum irisin levels and their possible variation with different body weights, % fat mass, insulin sensitivity and other obesity-related factors, 30 male rats were used. At weaning day (postnatal day 21), the animals were randomly divided into three groups: (1) control group, with ad libitum access to standard chow diet (ND = normal diet) (3.85 kcal/g; 10% kcal % fat; Research Diets, Inc.) (n = 10); (2) diet induced obesity (DIO) group, with ad libitum access to HFD diet (4.73 kcal/gm; 45% kcal % fat; Research Diets, Inc.) (n = 10); and (3) CR group (n = 10), with standard chow diet but the food was restricted to 65% of the daily amount ingested by the control group. To this CR group, a fixed amount of food was provided daily, and the animals ate all the food offered. The animals subjected to this feeding regimen had a lower percentage of body fat and increased sensitivity to insulin. The animals were housed under these feeding regimens for three months until they were sacrificed. Animals were sacrificed two hours after the beginning of the light phase at 10:00 a.m.
Alterations in FNDC5 gene expression and circulating irisin levels by fasting: Short-term experiments
We evaluated the impact of caloric restriction under a short-term duration. To this end, adult rats (BW: 260 g ± 4, 11 weeks of age) were fed with ND and subjected to fasting for 48 hours.
Effects of leptin in FNDC5 gene expression and circulating irisin levels in fed and fasted rats
To monitor changes in FNDC5 mRNA expression and circulating irisin in conditions of altered leptin signalling, adult rats (350 g ± 9) were fed ad libitum and received two intraperitoneal (IP) injections of leptin (Prospec, 1 mg/kg dissolved in 200 μl of saline) or saline (control group). Injections were applied 24-h and 2-h prior to animal sacrifice. Treatments started at 08:00 a.m. and were carried out in the light phase.
In an attempt to establish if leptin regulates hypothalamic and peripheral FNDC5 mRNA expression levels and plasma irisin levels in a nutritional-dependent fashion, we fed ad libitum one group of adult rats (189 g ± 9) and another group was deprived of food for 48 h. Fed (with normal values of insulin, leptin and glucose) and fasted (state associated with low levels of insulin, glucose and leptin) rats received two IP injections of leptin (Prospec, 1 mg/kg dissolved in 200 μl of saline) or vehicle. Injections were applied at 24-h and 48-h after the beginning of fasting. Treatments started at 08:00 a.m. and were carried out in the light phase. Animals were sacrificed 2-h after the 2nd injection (10:00 a.m.).
Effects of insulin and metformin in FNDC5 gene expression and circulating irisin levels
To evaluate the impact of insulin and insulin sensitizers in FNDC5 mRNA expression and plasma irisin levels, we treated rats fed with ND with insulin or metformin for 2 weeks. To this end, adult rats (290 g ± 2.2) were fed ad libitum. Insulin (Novo Nordisk Phama, 1 UI/kg, dissolved in 200 μl of saline and administrated IP), metformin (Sandoz, 300 mg/kg, dissolved in 200 μl of saline and administrated subcutaneously) and vehicle treatments were carried out in the light phase. Animals were sacrificed 2 h after the last injection (day 15).
Effects of irisin in serum insulin and glucose levels in fed rats
To monitor changes in circulating insulin and glucose levels in conditions of altered irisin, adult rats (197 g ± 3.5) were fed ad libitum and received two intraperitoneal (IP) injections of irisin (Phoenix, 20 μg/rat dissolved in 200 μl of saline) or saline (control group). Injections were applied 24-h and 2-h prior to animal sacrifice. Treatments started at 09:00 a.m. and were carried out in the light phase.
Effects of diabetes on FNDC5 gene expression and circulating irisin levels in fed and fasted rats
In order to assess the effect of diabetes on FNDC5 mRNA expression and circulating irisin levels, two new experiments were conducted in which we induced diabetes in adult rats (274 g ± 3.2) by a single injection of alloxan (Sigma-Aldrich, 130 mg/kg dissolved in 200 μl of saline and administrated IP). In one of them, six days after alloxan injection a group of rats was fasted for 48 h to reduce glucose levels. The animals were sacrificed 8 days after alloxan injection.
In the other experiment, nine days after alloxan injection part of the rats were treated with subcutaneous injections of exogenous human NPH insulin to maintain glycemia in these animals as close to the normoglycemia as possible. The glucose levels were measured every day at 12:00 h and 20: 00 h with a glucometer and a blood drop from the tail vein. The normal dose of NPH insulin was 9.5 U/day but the daily dose of insulin was adjusted according to the glycemia of each animal. Two units of insulin were injected at 13:00 h and the 7.5 remaining units at 21:00 h. The control group received saline solution. The animals were sacrificed 8 days after insulin treatment.
Statistical analysis
Data were analysed using SigmaStat 3.1 (Systat Software, Inc.) and are presented as the means ± SEM. Statistical significance was determined by t-tests (experiments with two groups), one-way ANOVA with post hoc Tukey’s tests (experiments with more than two groups and one variable) or two-way ANOVA with post hoc Tukey’s tests (experiments with more than two groups and two variables). P < 0.05 was considered significant. Different letters or symbols above bars indicate statistical significance.
Results
Tissue distribution and differential expression of the FNDC5 gene in rats
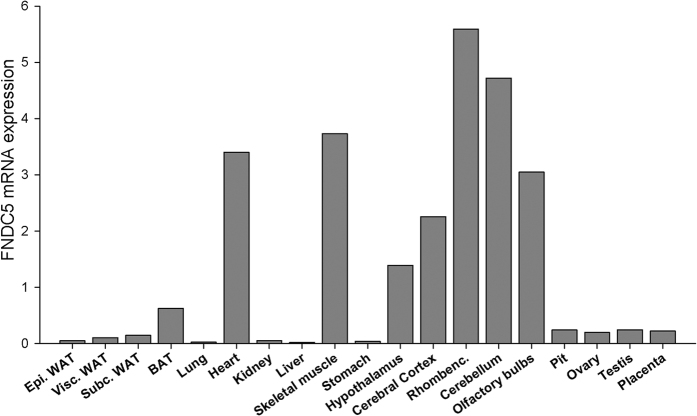
As shown in Fig. 1, FNDC5 mRNA is prominently expressed in different brain areas and muscle (both cardiac and skeletal). Brown adipose tissue, pituitary, placenta, testis and ovary also showed relatively high expression levels with modest expression in the depots of white adipose tissue. Other tissues such as lung, kidney, liver, and stomach had very low or undetectable FNDC5 mRNA levels.
Figure 1. Expression profile of the FNDC5 gene in different tissues in the adult rat.
A panel of tissues from adult male and female (ovary and placenta) rats was screened for expression of FNDC5 mRNAs, using real-time RT-PCR.
Alterations in FNDC5 gene expression and circulating irisin levels in rats reared with different diets: Long-term experiments
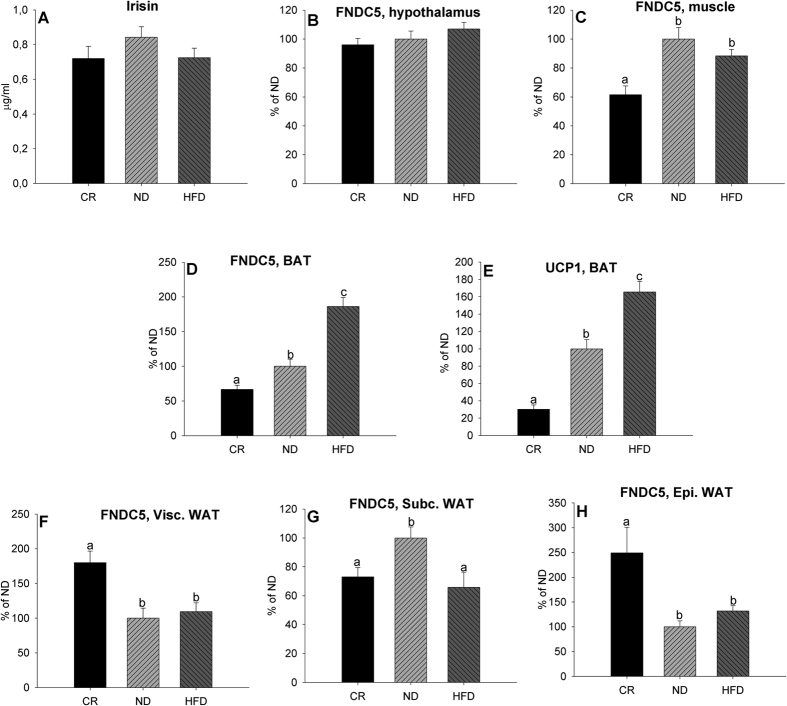
Corporal and some plasmatic parameters of rats reared for three months with different diets were published previously25. The % fat mass was very low in animals reared in CR (scarcely a 2%) and increased to 9.5% in the control group and 18.8% in animals with HFD (Table 1). The animals subjected to CR displayed signs of increased insulin sensitivity because they had the lowest basal glucose and insulin levels. In contrast, HFD rats presented signs of insulin resistance and metabolic deregulation, as evidenced by higher basal glucose, insulin, and leptin and increased plasma triglyceride concentrations (Table 1)25. To monitor if metabolic alterations induced by the different diets influence circulating irisin levels and FNDC5 gene expression, we analysed serum irisin values and FNDC5 mRNA expression by real-time RT-PCR. As shown in Fig. 2A, animals subjected to CR and HFD displayed diminished serum irisin levels but the differences were not statistically significant. No changes were observed in hypothalamic (Fig. 2B) FNDC5 mRNA expression. The expression profiles of FNDC5 in muscle (Fig. 2C) and inguinal subcutaneous WAT (Fig. 2G) were similar to the circulating levels of irisin, with lower values in the CR in both tissues and in subcutaneous WAT in animals fed with HFD. Levels of FNDC5 and UCP1 (uncoupling protein 1) gene expression in BAT (Fig. 2D,E) increased in proportion to the percentage of fat mass and FNDC5 gene expression was enhanced with CR in visceral (Fig. 2F) and epididymal (Fig. 2H) WAT.
Table 1. Effect of caloric restriction and high fat diet during 3 months on % fat mass, % lean mass, body weight gain, and plasma glucose, insulin, leptin and triglycerides levels.
| CR | ND | HFD | |
|---|---|---|---|
| Fat mass (%) | 2.1 ± 0.6 a | 9.5 ± 0.5 b | 18.8 ± 0.9 c |
| Lean mass (%) | 80.3 ± 0.7 a | 73.1 ± 0.7 b | 66.2 ± 0.8 c |
| Body weight gain (g) | 173.2 ± 4.8 a | 306.8 ± 9.0 b | 362.0 ± 7.3 c |
| Glucose (mg/dl) | 72.0 ± 1.4 a | 78.44 ± 1.0 b | 139.3 ± 4.0 c |
| Insulin (ng/ml) | 1.01 ± 0.1 a | 3.2 ± 0.3 b | 4.1 ± 0.3 c |
| Leptin (ng/ml) | 3.3 ± 0.6 a | 8.5 ± 1.0 b | 15.7 ± 0.8 c |
| Tryglicerides (mg/dl) | 64.1 ± 3.9 a | 120.8 ± 6.2 b | 148.1 ± 8.3 c |
Values are expressed as mean ± SEM. Different letters indicate statistical differences (One-way ANOVA with post hoc Tukey test, a value of P < 0.05 was considered statistically significant). CR: caloric restriction (n = 10), ND: normal diet (n = 10) and HFD: high fat diet (n = 10). The animals were reared in these diets during three months after weaning.
Figure 2.
Effect of the HFD and CR for three months on plasma irisin (A) levels, FNDC5 expression levels in the hypothalamus (B), muscle (C), BAT (D) and visceral (F), subcutaneous (G) and epididymal (H) white adipose tissue of adult male rats and UCP1 expression levels in BAT (E). Values are expressed in μg/ml for circulating irisin and arbitrary units for FNDC5 and UCP1 gene expression, as the mean ± SEM, where ND = 100%. Different letters above the bars indicate statistical differences (One-way ANOVA with post hoc Tukey’s test, a value of P < 0.05 was considered statistically significant). CR: caloric restriction (n = 10), ND: normal diet (n = 10) and HFD: high fat diet (n = 10). The animals were reared on these diets for three months after weaning.
Alterations in FNDC5 gene expression and circulating irisin levels in fasted rats: short-term experiments
Main body parameters and plasma levels of key factors in rats fasted for 48 h are shown in Table 2. As expected, BW gain and glucose, insulin, and triglyceride plasma levels diminished with fasting. The epididymal-somatic index did not change with the fast, while the percentage of visceral fat and hepatic somatic index decreased in fasted rats (Table 2).
Table 2. Effect of fasting during 48-h on body weight gain, plasma glucose, insulin and triglycerides levels and somatic indices.
| Body weight gain (g) | Glucose (mg/dl) | Insulin (ng/ml) | Triglycerides (mg/dl) | Somatic index. Liver (g/100 g BW) | Somatic index. Epididymal (g/100 g BW) | Somatic index. Visceral (g/100 g BW) | |
|---|---|---|---|---|---|---|---|
| Fed | 41.2 ± 2.5 | 120 ± 1.5 | 2.3 ± 0.3 | 162 ± 13 | 3.7 ± 0.1 | 0.96 ± 0.05 | 0.68 ± 0.04 |
| Fast | 11.6 ± 4.4* | 91 ± 1.1* | 0.44 ± 0.07* | 68 ± 4.7* | 2.6 ± 0.08* | 0.88 ± 0.05 | 0.49 ± 0.05* |
Values are expressed as mean ± SEM. *P < 0.05 vs. fed, t-test, n = 6. Somatic index was calculated as the ratio between tissues weight and body weight and was expressed as g/100 g body weight (BW).
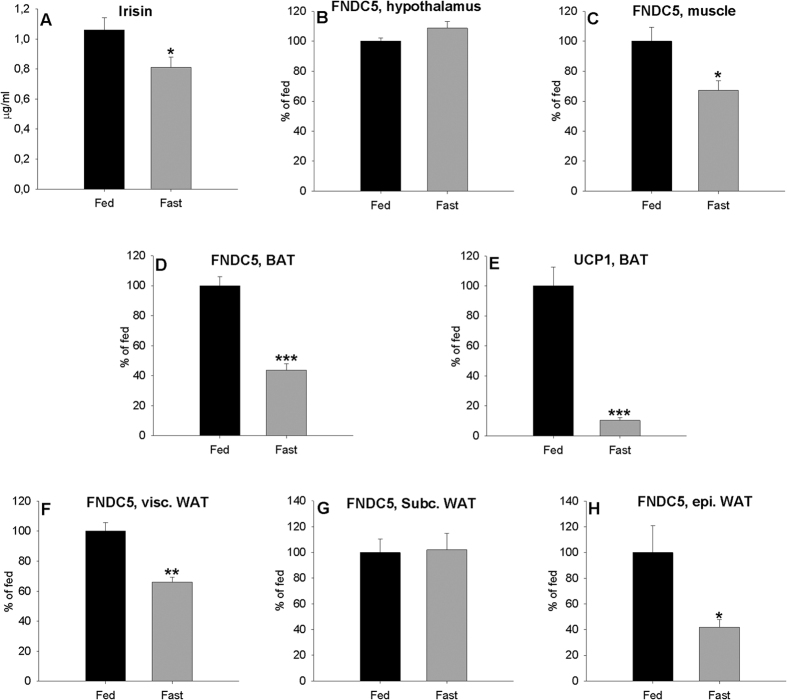
The results FNDC5 mRNA expression and circulating irisin levels during fasting are shown in Fig. 3. Interestingly, a 48-h fast led to a decrease in serum irisin levels (Fig. 3A) and reduced FNDC5 mRNA levels in muscle (Fig. 3C), BAT (Fig. 3D) and in visceral and epididymal white adipose tissue (Fig. 3F,H). Values of FNDC5 mRNA in subcutaneous WAT did not change (Fig. 3G). UCP1 expression levels in BAT (Fig. 3E) decreased significantly with fasting, just like did FNDC5 values. In the hypothalamus, the effect of fasting was contrary although the difference was not statistically significant (P > 0.05) (Fig. 3B). These results suggest that fasting has different effects on central and peripheral FNDC5 gene expression.
Figure 3.
Effect of 48 h of fasting on plasma irisin (A) levels, FNDC5 expression levels in the hypothalamus (B), muscle (C), BAT (D) and visceral (F), subcutaneous (G) and epididymal (H) white adipose tissue and UCP1 expression levels in BAT (E) of adult male rats. Values are expressed in μg/ml for circulating irisin and arbitrary units for FNDC5 and UCP1 gene expression, as the mean ± SEM, where fed = 100%. N = 6. Different symbols above the bars indicate statistical differences (t-test, a value of P < 0.05 was considered statistically significant). *P < 005 vs. fed.
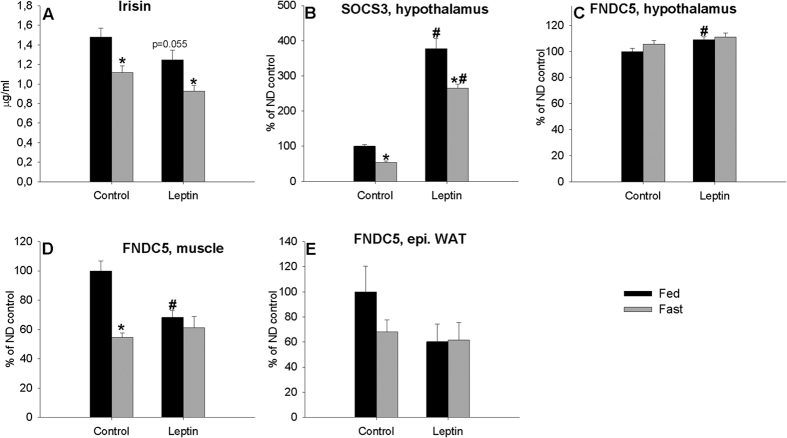
Effects of leptin on FNDC5 gene expression and circulating irisin levels
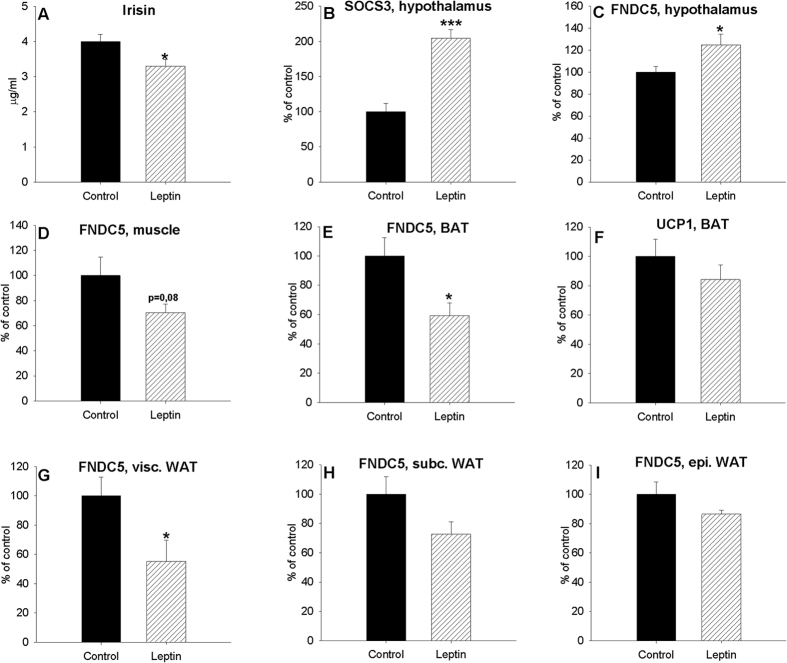
The efficiency of the treatment was corroborated by the increased expression of hypothalamic suppressor of cytokine signalling 3 (SOCS3) (Fig. 4B), a primary target of leptin with a negative feedback regulation26. Interestingly, leptin treatment was associated with increased hypothalamic FNDC5 expression levels (Fig. 4C) but decreased serum irisin levels (Fig. 4A) like muscle (Fig. 4D), BAT (Fig. 4E) and visceral (Fig. 4G), subcutaneous (Fig. 4H) and epididymal (Fig. 4I) white adipose tissue FNDC5 gene expression. These results suggest that leptin has different effects on central and peripheral FNDC5 gene expression, which is similar to the effects of fasting. Unlike FNDC5 gene expression, no changes were observed in BAT UCP1 mRNA expression levels after treatment with leptin (Fig. 4F).
Figure 4.
Effect of 2-day IP leptin treatment in fed animals on plasma irisin (A) levels, SOCS3 gene expression (B), FNDC5 expression levels in the hypothalamus (C), muscle (D), BAT (E) and visceral (G), subcutaneous (H) and epididymal (I) white adipose tissue of adult male rats and UCP1 expression levels in BAT (F). Values are expressed in μg/ml for circulating irisin and arbitrary units for FNDC5 and SOCS3 gene expression, as the mean ± SEM, where control = 100%. n = 6–7. *, ***P < 0.05 and 0.01 respectively vs. control (rats injected with saline) (t-test).
In order to corroborate the previous results and to assess if leptin regulates hypothalamic and peripheral FNDC5 mRNA expression levels and plasma irisin levels in a nutritional-dependent fashion we repeated the last experiment but in fed and fasted rats. The results are showed in Fig. 5.
Figure 5.
Effect of 2-day IP leptin treatment in fed and fasted animals on plasma irisin levels (A), SOCS3 gene expression (B) and FNDC5 expression levels in the hypothalamus (C), muscle (D), and epididymal (E) white adipose tissue of adult male rats. Values are expressed in μg/ml for circulating irisin and arbitrary units for FNDC5 and SOCS3 gene expression, as the mean ± SEM, where fed animals treated with saline were the controls = 100%. n = 6–7. Different symbols above bars indicate statistical differences (Two-way ANOVA with post hoc Tukey’s test, a value of P < 0.05 was considered statistically significant). *P < 005 vs. fed; #P < 0.05 vs. saline.
Overall, there was a significant (P < 0.001 fed vs. fast and P = 0.031 vehicle vs. leptin, two-way ANOVA) effect of fasting and leptin treatment on plasma irisin levels but no significant (P > 0.05, two-way ANOVA) effect of leptin treatment × status nutritional interaction, although the comparisons for leptin treatment within fed animals had a P = 0.055, similar to the diminished irisin levels in fasting animals in the previous experiment (Fig. 5A). In the hypothalamus, SOCS3 gene expression increased in all animals treated with leptin and diminished with fasting (Fig. 5B). In concordance with the previous results, FNDC5 mRNA expression levels diminished in muscle tissue of animals treated with leptin but this effect disappeared with fasting (Fig. 5D). Similar results were obtained for the hypothalamus, where FNDC5 mRNA expression levels increased in fed animals treated with leptin; however, this effect was not present in fasted animals (Fig. 5C).
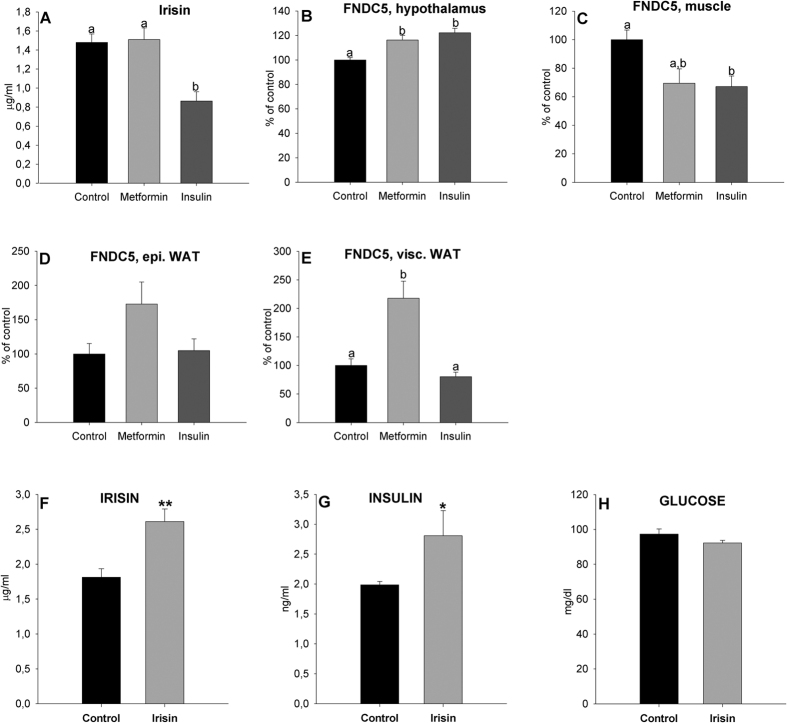
Effects of insulin and metformin on FNDC5 gene expression and circulating irisin levels
As expected, insulin and metformin injections diminished glucose levels demonstrating their efficacy (Table 3). Insulin and metformin treatments provoked a lower body weight gain with respect to controls; however, epididymal and visceral WAT mass were unchanged in insulin treated rats. With metformin, epididymal somatic index diminished with respect to controls and insulin treated rats. The hepatosomatic index and plasma triglyceride levels were reduced in insulin-treated rats (Table 3). Plasma irisin levels diminished in animals treated with insulin (Fig. 6A). Hypothalamic FNDC5 mRNA expression levels increased with insulin and metformin treatments (Fig. 6B). Muscle FNDC5 mRNA expression levels diminished in rats treated with insulin and metformin with respect to their controls, although with metformin the differences were not statistically significant (Fig. 6C). WAT FNDC5 gene expression increased with metformin (Fig. 6D,E), however in epididymal WAT (Fig. 6D) the differences were again not significant.
Table 3. Effect of a 14-day IP saline and insulin and subcutaneous metformin treatment on body weight gain, and plasma glucose and triglycerides levels and somatic index.
| Body weight gain (g) | Glucose (mg/dl) | Triglycerides (mg/dl) | Somatic index. Liver (g/100 g BW) | Somatic index. Epididymal (g/100 g BW) | Somatic index. Visceral (g/100 g BW) | |
|---|---|---|---|---|---|---|
| Control | 50.0 ± 1.8 a | 131.8 ± 4.7 a | 116.6 ± 6.5 a | 3.5 ± 0.1 a | 1.57 ± 0.09 a | 1.0 ± 0.06 |
| Metformin | 20.7 ± 7.4 b | 91.0 ± 11.5 b | 97.5 ± 10.5 a,b | 3.3 ± 0.2 a,b | 1.20 ± 0.03 b | 0.9 ± 0.06 |
| Insulin | 41.4 ± 1.6 c | 70.5 ± 10.5 b | 69.76 ± 4.84 b | 3.0 ± 0.1 b | 1.63 ± 0.12 a | 1.0 ± 0.04 |
Values are expressed as mean SEM. Different letters indicate statistical differences by treatment (One-way ANOVA with post hoc Tukey test, a value of P < 0.05 was considered statistically significant). N = 6 (metformin treatment) or 8 (saline and insulin treatments). Somatic index was calculated as the ratio between tissues weight and body weight and was expressed as g/100 g body weight (BW).
Figure 6.
Effect of a 14-day IP saline and insulin and subcutaneous metformin treatment on plasma irisin levels (A) and FNDC5 expression levels in the hypothalamus (B), muscle (C) and epididymal (D) and visceral (E) WAT of adult male rats and effect of 2-day IP irisin treatment in fed animals on plasma irisin (F), insulin (G) and glucose levels (H). Different letters above the bars indicate statistical differences (One-way ANOVA with post hoc Tukey’s test, a value of P < 0.05 was considered statistically significant). ***P < 0.05 and 0.01 respectively vs. control (rats injected with saline) (t-test). Values are expressed as the mean ± SEM where animals treated with saline. N = 6 (metformin treatment), 8 (saline and insulin treatments) and 7 (irisin treatment). Values are expressed in mg/dl for plasma glucose levels, ng/ml for plasma insulin levels and μg/ml for circulating irisin levels. Arbitrary units were used for FNDC5 gene expression where rats injected with saline = 100%.
Effects of irisin in serum insulin and glucose levels in fed rats
Exogenous irisin treatment caused a physiologic increase in circulating irisin levels (Fig. 6F). In turn, irisin caused an increase in the amount of circulating insulin (Fig. 6G) without altering blood glucose levels (Fig. 6H).
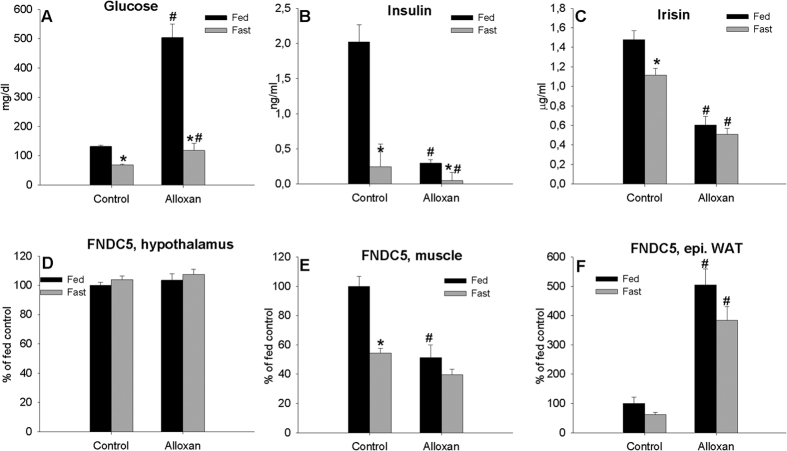
Effects of diabetes on FNDC5 gene expression and circulating irisin levels
As expected, blood glucose levels in both fed and fasted rats treated with alloxan were higher compared with their controls (Fig. 7A). When fed rats showed blood glucose levels higher than 260 mg/dl were classified as diabetic. Insulin levels diminished significantly in animals treated with alloxan and with fasting (Fig. 7B). Alloxan treatment diminished the percentage of visceral and epididymal WAT considerably both in fed and fasted rats (data not shown). Plasma irisin levels diminished with alloxan in fed and fasted animals with respect to their controls (Fig. 7C). Fasting was also associated with reduced circulating levels of irisin (as we observed in experiment 3), but only in control animals (Fig. 7C). The FNDC5 gene showed a similar expression profile in muscle (Fig. 7E). However, epididymal WAT FNDC5 mRNA expression levels increased with alloxan treatment in fed and fasted rats (Fig. 7F). No differences were observed in hypothalamic FNDC5 gene expression (Fig. 7D). Again, muscle FNDC5 mRNA expression levels diminished with fasting but only in control rats (Fig. 7E). Diabetes also diminished FNDC5 gene expression but this effect was dependent of nutritional status. Independently of feeding, WAT FNDC5 gene expression increased substantially with alloxan treatment (Fig. 7F).
Figure 7.
Effect diabetes induced by a single injection of alloxan in fed and fasted (48 h) animals on plasma glucose (A) insulin (B) and irisin (C) levels and FNDC5 gene expression levels in the hypothalamus (D), muscle (E) and epididymal (F) WAT of adult male rats. *P < 0.05 vs. fed animals. #P < 0.05 vs. saline. (Two-way ANOVA with post hoc Tukey’s test, a value of P < 0.05 was considered statistically significant). Values are expressed in mg/dl for plasma glucose levels, ng/ml for plasma insulin levels and μg/ml for circulating irisin levels. Arbitrary units were used for FNDC5 gene expression, as the mean ± SEM, where rats fed and injected with saline = 100%. N = 8 (saline treatment) or 10 (alloxan treatment).
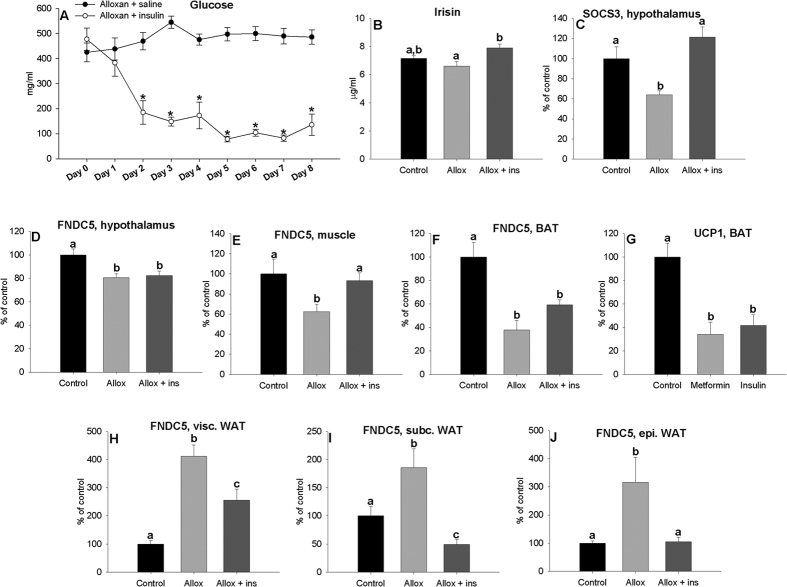
In the next experiment, we tested if parameters altered by alloxan-induced diabetes can be reversed by insulin therapy. As in the previous experiment, the alloxan treatment induced a robust increase in blood glucose levels nine days after alloxan injection (Fig. 8A). Glucose levels dropped below 200 mg/dl 48 h after beginning insulin treatment, and after 5 days normoglycemic levels were maintained until the animals were sacrificed (Fig. 8A). No changes were observed in plasma irisin levels of animals treated with alloxan with respect to the control group; however they showed lower levels than the rats subjected to insulin therapy (Fig. 8B). Hypothalamic SOCS3 (Fig. 8C) and FNDC5 (Fig. 8D) mRNA expressions decreased in diabetic rats. But unlike FNDC5 gene expression, SOCS3 mRNA expression levels were reversed by insulin treatment. FNDC5 mRNA expression was reduced in muscle (Fig. 8E) and BAT (Fig. 8F) in diabetic rats. UCP1 gene expression in BAT (Fig. 8G) showed exactly the same pattern that FNDC5 mRNA expression levels, reinforcing the hypothesis that UCP1 and FNDC5 are related. In muscle, the values were recovered by insulin treatment. In all WAT depots studied, FNDC5 gene expression enhanced with diabetes, and these values decreased significantly (even over control values) after insulin treatment (Fig. 8H–J).
Figure 8.
Effect of treatment of diabetic rats using 9.5 IU/day of NPH insulin for 8 days on plasma glucose levels (A) and effect after 8-day treatments of diabetics with insulin on plasma irisin (B) levels and SOCS3 (C), FNDC5 expression levels in the hypothalamus (D), and FNDC5 expression levels in muscle (E), BAT (F) and visceral (H), subcutaneous (I) and epididymal (J) white adipose tissue of adult male rats and UCP1 expression levels in BAT (G). *P < 0.05 vs. diabetics rats injected with saline. Different letters above the bars indicate statistical differences (One-way ANOVA with post hoc Tukey’s test, a value of P < 0.05 was considered statistically significant). Values are expressed in mg/dl for plasma glucose levels and μg/ml for circulating irisin levels. Arbitrary units were used for FNDC5 and SOCS3 gene expression, as the mean ± SEM, where normal rats injected with saline = 100%. N = 8.
Discussion
Initially irisin was described as a myokine that induces white fat “browning” in vivo and in vitro12, and later as an adipokine16. Since its discovery there have been numerous published studies about its regulation and function. Böstrom et al. proposed that FNDC5 was cleaved producing the soluble protein irisin that increases total body energy expenditure and resistance to obesity-linked insulin-resistance, and that this effect was a result of browning produced by irisin in adipose tissue12. Both in humans and rodents correlated brown fat tissue formation with anti-obesity effects, suggesting a central role of beige adipocyte thermogenesis in whole-body energy metabolism27,28,29. However, controversy has emerged regarding the association between irisin and obesity or metabolic disorders. Some studies have suggested that irisin ameliorates obesity, glucose disorders and insulin resistance14,30,31 and that circulating irisin is negatively associated with BMI and percentage of fat mass14,32; however, other studies have found that irisin is positively correlated with these parameters33,34 while others have revealed no correlation between irisin and BMI35,36,37.
To elucidate the relationship between serum irisin levels and FNDC5 gene expression in different tissues, we evaluated blood irisin levels and FNDC5 mRNA expression levels in the hypothalamus, gastrocnemius muscle, brown adipose tissue and different depots of WAT [visceral (abdominal), epididymal and subcutaneous] in different models of altered metabolism and moreover we studied the effects of exogenous irisin on insulin levels.
To begin, we studied the distribution of FNDC5 mRNA expression in different rat tissues. As previously reported, FNDC5 mRNA was expressed in high levels in muscles20,34 and also in all brain regions analysed18,38 suggesting a role for FNDC5/irisin in the brain. This theory is supported by different reports of FNDC5/irisin function in different brain processes. Endurance exercise enhances FNDC5 gene expression in the hippocampus of mice which in turn stimulates BDNF gene expression and forces expression of FNDC5 in primary cortical neurons increasing cell survival20. In humans, irisin was detected in cerebrospinal fluid and hypothalamic sections, especially paraventricular neurons, colocalising with neuropeptide Y21. We also reported high levels of FNDC5 mRNA expression in BAT, which could be due to the prominent role of BAT in the release of heat through the action of UCP1 and in the control of glucose and lipid metabolism39 since cold exposure increases circulating irisin levels40 in addition to irisin driving brown-fat-like development of white fat and thermogenesis stimulating UCP1 expression12. In this work, we demonstrated that the FNDC5 and UCP1 gene expression patterns are identical in BAT during chronic caloric restriction, fasting, feeding with HFD and treatments with alloxan or alloxan plus insulin. These results are supported by other studies in rodents demonstrating that HFD increases brown adipose tissue UCP1 mRNA and protein content41 and that fasting diminishes UCP1 gene and protein expression42. We observed that alloxan treatment clearly reduced FNDC5 expression in BAT, which could be explained by a reduced thermogenic capacity of BAT as noted in animal studies with chronic insulin deficiency43,44. Furthermore, mice with type 1 diabetes mellitus showed a reversion of glucose homeostasis to normalcy increasing BAT quantity by transplants45. After acute leptin treatment in fed rats, we observed no differences in BAT UCP1 mRNA, as is consistent with previous reports42,46; however, FNDC5 mRNA expression was reduced in these conditions. Sivitz et al. observed that leptin effects on UCP1 mRNA and protein levels may be discordant42, therefore it cannot be ruled out that our discrepancies are due to the tendency for UCP1 mRNA and protein expression levels after leptin treatment to be different or even that this also occurs with FNDC5. The relatively high expression of FNDC5 in the reproductive neuroendocrine axis (hypothalamus, pit, ovary, testis and placenta) suggests an effect of irisin on reproductive function. However, additional studies are required to clarify whether such a relationship exists.
It must be borne in mind that different WAT depots may differ in physiological function. Excess accumulation of visceral adipose in the abdominal cavity is associated with metabolic syndrome, diabetes, cardiovascular disease and mortality47. However, in rodents, subcutaneous WAT, appears to be relatively benign and transplantation of subcutaneous adipose tissue from normal48 or exercise-trained donor49 to visceral cavity can improve glucose metabolism and insulin sensitivity. Irisin may promote maintenance of “healthy” subcutaneous adipose because FNDC5 is induced by exercise in this depot and increases brown/beige fat cells12. Although WAT FNDC5 gene expression may represent only a small fraction of that expressed in muscle, brain, or even brown adipose tissue, Moreno-Navarrete et al. suggested that it is the adipose tissue (studies realized with visceral and subcutaneous WAT) and not the skeletal muscle expression that correlates with levels of circulating irisin in humans14. In support, Yang et al. demonstrated that FNDC5 protein expression in adipose tissue, not skeletal muscle, contributed to the change of circulating irisin in HFD-induced obese mice50; but, Yang et al. only analysed subcutaneous abdominal adipose tissue. However, the existence of diverse fat depot states in rats offers the possibility of differential contributions from these depots to circulating irisin levels. To explore this, we analysed FNDC5 gene expression in epididymal, visceral (abdominal) and subcutaneous WAT and BAT during different states of metabolic distress. We observed that the alterations in blood irisin levels resulting from changes in the feeding depend on the duration and nature of the changes. In long-term experiments, neither caloric restriction nor high fat diet cause changes in the circulating irisin levels, however with 48 hours of fasting, blood irisin levels drop along with the decline observed in FNDC5 mRNA expression levels in muscle, BAT and visceral and epididymal adipose tissue. However, changes were not observed in subcutaneous fat, suggesting that the changes produced in circulating irisin levels with short-term restriction caloric are not related to FNDC5 gene expression levels in subcutaneous fat, but could be related to variations in muscle, BAT or other white fat depots such as visceral or epididymal. During long-term experiments, BAT FNDC5 mRNA expression levels increased proportionally to the percentage of fat mass. In WAT after three months with CR, FNDC5 mRNA expression levels increased in epididymal and visceral adipose tissue but diminished with CR and HFD in subcutaneous fat, demonstrating differences in FNDC5 expression depending on the adipose tissue depot. We suggest that the increase observed in epididymal and visceral WAT FNDC5 expression, or at least partially, during CR occurs in an attempt to compensate for the decrease that occurs in the mass of those deposits, which were more affected by CR than the subcutaneous WAT51. This hypothesis is reinforced by the increase in FNDC5 mRNA expression levels in all WAT depots in diabetic rats, concomitant with a significant decrease in their fat mass, which even disappears (data not shown). We suggest that circulating irisin levels are a reflection of the combination of irisin released from various fat depots and from muscle, and that when the amount of WAT decreases substantially, FNDC5 levels in these depots increase to attempt to compensate for the tissue loss, which may explain some of the observed discrepancies between insulin levels and FNDC5/irisin, such as metformin treatment or long-term caloric restriction.
Hypothalamic FNDC5 expression did not change with any of the tested diets suggesting that its regulation does not depend on alimentation/nutrition. In this work, we observed that an acute leptin treatment increased hypothalamic FNDC5 transcript levels and diminished their levels in gastrocnemius, BAT and visceral adipose tissue correlating nicely with the observed decline in circulating irisin levels. However, these differences disappeared in rats that had fasted for 48 h suggesting that peripheral leptin regulates hypothalamic and peripheral FNDC5 gene expression in a nutritional-dependent fashion. In a previous work, Rodriguez et al. observed that leptin administration was associated with increased gastrocnemius FNDC5 transcript levels, a slight increase in circulating irisin in mice and downregulated FNDC5 expression in murine differentiated subcutaneous adipocytes52. Additional studies are required to identify the source of the discrepancies between different experiments, including the experimental design and animal model.
Numerous studies have reported a positive association of muscle FNDC5/irisin with insulin resistance, speculating on the negative desensitizing effects of irisin on insulin action16,34,53. However, the results of our study also support a relationship between insulin production and the levels of circulating irisin. Acute treatments with exogenous irisin enhanced circulating insulin levels displaying a relationship between irisin and insulin production. Moreover, situations with low levels of insulin including fasting and CR and treatments with leptin, alloxan, metformin or insulin (which decreases endogenous insulin production) are associated with reduced FNDC5 gene expression levels in muscle but also reduced circulating irisin levels in most cases. These results are consistent with a recent study demonstrating that serum irisin concentrations were lower in type 2 diabetes mellitus patients and increased after continuous subcutaneous insulin54.
Two recent works in mouse showed that metformin promotes FNDC5 gene expression and irisin release from skeletal muscle55,56. The differences with respect to this work could be due to the species, mice vs. rats and/or also to the effects of the metformin in the weight and body composition. In our case, the treatment with metformin resulted in a sharp decrease in both body weight and fat mass gain, but not in the mentioned works where no variations were observed on body weight of wild-type mice treated with metformin. Supporting this theory Li et al. found that after 6 months of metformin treatment, there was a significant decrease in % fat mass, body weight and circulating irisin in polycystic ovary syndrome women57. However, to clarify this point further studies are needed, where the body weight gain do not change between controls and rats treated with metformin.
Doubts have been raised about circulating irisin, and there are limitations of the irisin assay22,58. We cannot rule out that the ELISA assays used in this study detected false signals from cross-reacting proteins. However, Jedrychowski et al. using tandem mass spectrometry have unequivocally shown that human irisin exists, circulates, and is regulated by exercise23. Moreover, they used immunoblotting of irisin plasma samples with a polyclonal antibody from the same commercial source as that used in the ELISA assays in this work and detected a band at ≈12 kDa, the predicted size of the irisin polypeptide23, suggesting that this antibody works efficiently. A strong point in our work is that several of the experiments, fasting and leptin and alloxan treatments are repeated and the results are replicable for FNDC5 gene expression and serum irisin levels with rare exceptions in alloxan experiments that can be explained by the variable duration between the two treatments of alloxan.
In conclusion, FNDC5 mRNA was expressed in high levels in muscles, brain and in the reproductive neuroendocrine axis. We demonstrated for the first time that there are differences in FNDC5 expression depending on the WAT depot and when the amount of one of these depots decreases considerably, FNDC5 mRNA expression increases, probably as a compensatory effect. Serum irisin levels diminish after a 48-h fast and with leptin, insulin and alloxan treatments, but no changes were observed during long-term experiments with different diets. We suggested that the combination of different depots of adipose tissue and skeletal muscle expression correlates with circulating irisin levels. On the other hand, hypothalamic FNDC5 expression did not change for any of the tested diets suggesting that their regulation is not dependent on alimentation but was increased with leptin, insulin and metformin treatments. These results suggest that the regulation of central and peripheral FNDC5/irisin expression are different and have different functions. However, additional experiments are required to characterize such functions.
Additional Information
How to cite this article: Varela-Rodríguez, B. M. et al. FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Sci. Rep. 6, 29898; doi: 10.1038/srep29898 (2016).
Acknowledgments
The results of this work have been funded by the Projects Nº: EM2013/011 to S.S.A. integrated in Plan I2C and funded by Consellería de Cultura, Educación e Ordenación Universitaria (Xunta de Galicia, Spain) and PI13/00322 to F.C. integrated in the National Plan for Scientific Research, Development and Technological Innovation 2013-2016 and funded by the ISCIII, General Subdirection of Assesment and Promotion of the Research, European Regional Development Fund (FEDER) “A way of making Europe”.
Footnotes
Author Contributions B.M.V.-R. conducted the experimental studies and analytical procedures and evaluated the data; L.P.-B., P.J.-V. and B.V.-B. assisted in the conduction of the experimental studies and analytical techniques; F.C., helped in design of the study, evaluated part of the data and revised the manuscript; S.S.-A. designed the study, revised and analyzed the data, and wrote the manuscript. S.S.-A. takes full responsibility for the work as a whole.
References
- Organization W. H. Diabetes fact sheet no. 312. Available at http://wwwwhoint/mediacentre/factsheets/fs312/en/indexhtml (2011).
- Travers M. E. & McCarthy M. I. Type 2 diabetes and obesity: genomics and the clinic. Hum Genet 130, 41–58 (2011). [DOI] [PubMed] [Google Scholar]
- McCarthy M. I. Genomics, type 2 diabetes, and obesity. N Engl J Med 363, 2339–2350 (2010). [DOI] [PubMed] [Google Scholar]
- Williams L. M. Hypothalamic dysfunction in obesity. Proc Nutr Soc 71, 521–533 (2012). [DOI] [PubMed] [Google Scholar]
- Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab 24, 40–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt B. F. & Bruning J. C. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci 1212, 97–113 (2010).21070248 [Google Scholar]
- Belgardt B. F. et al. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci USA 107, 6028–6033 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122, 153–162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima R. S. & Lazar M. A. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol 22, 1023–1031 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima R. S., Qi Y., Singhal N. S., Jackson M. B. & Scherer P. E. Brain adipocytokine action and metabolic regulation. Diabetes 55 Suppl 2, S145–154 (2006). [DOI] [PubMed] [Google Scholar]
- Chen H. C., Roth J. D., Schroeder B. E. & Weyer C. Role of islet-, gut-, and adipocyte-derived hormones in the central control of food intake and body weight: implications for an integrated neurohormonal approach to obesity pharmacotherapy. Curr Diabetes Rev 4, 79–91 (2008). [DOI] [PubMed] [Google Scholar]
- Bostrom P. et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger H. et al. Common Genetic Variation in the Human FNDC5 Locus, Encoding the Novel Muscle-Derived ‘Browning’ Factor Irisin, Determines Insulin Sensitivity. PLoS One 8, e61903 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Navarrete J. M. et al. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association With Obesity and Insulin Resistance. J Clin Endocrinol Metab 94, E769–778 (2013). [DOI] [PubMed] [Google Scholar]
- Chen J. Q., Huang Y. Y., Gusdon A. M. & Qu S. Irisin: a new molecular marker and target in metabolic disorder. Lipids Health Dis 14, 1–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Rivada A. et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One 8, e60563 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi M. S. et al. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 231, 296–304 (2013). [DOI] [PubMed] [Google Scholar]
- Forouzanfar M. et al. Fndc5 overexpression facilitated neural differentiation of mouse embryonic stem cells. Cell Biol Int 39, 629–637 (2015). [DOI] [PubMed] [Google Scholar]
- Moon H. S., Dincer F. & Mantzoros C. S. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 62, 1131–1136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann C. D. et al. Exercise Induces Hippocampal BDNF through a PGC-1alpha/FNDC5 Pathway. Cell Metab 18, 649–659 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piya M. K. et al. The identification of irisin in human cerebrospinal fluid: influence of adiposity, metabolic markers, and gestational diabetes. Am J Physiol Endocrinol Metab 306, E512–518 (2014). [DOI] [PubMed] [Google Scholar]
- Albrecht E. et al. Irisin - a myth rather than an exercise-inducible myokine. Sci Rep 5, 8889 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski M. P. et al. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab 22, 734–740 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiao-Alvarellos S. et al. Changes in Hypothalamic Expression of the Lin28/let-7 System and Related MicroRNAs During Postnatal Maturation and After Experimental Manipulations of Puberty. Endocrinology 154, 942–955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiao-Alvarellos S., Pena-Bello L., Manfredi-Lozano M., Tena-Sempere M. & Cordido F. Perturbation of Hypothalamic MicroRNA Expression Patterns in Male Rats After Metabolic Distress: Impact of Obesity and Conditions of Negative Energy Balance. Endocrinology 155, 1838–1850 (2014). [DOI] [PubMed] [Google Scholar]
- Morris D. L. & Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab 297, E1247–1259 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussnitzer M. et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N Engl J Med 373, 895–907 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P. et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121, 96–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156, 304–316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X. Q. et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta 1852, 1867–1875 (2015). [DOI] [PubMed] [Google Scholar]
- Liu T. Y. et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci (Lond) 129, 839–850 (2015). [DOI] [PubMed] [Google Scholar]
- Choi Y. K. et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract 100, 96–101 (2013). [DOI] [PubMed] [Google Scholar]
- Moreno M. et al. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PLoS One 10, e0124100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J. Y. et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 61, 1725–1738 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Gomar F. et al. Circulating irisin levels are not correlated with BMI, age, and other biological parameters in obese and diabetic patients. Endocrine 46, 674–677 (2014). [DOI] [PubMed] [Google Scholar]
- Timmons J. A., Baar K., Davidsen P. K. & Atherton P. J. Is irisin a human exercise gene? Nature 488, E9–10; discussion E10–11 (2012). [DOI] [PubMed] [Google Scholar]
- Quinones M., Folgueira C., Sanchez-Rebordelo E. & Al-Massadi O. Circulating Irisin Levels Are Not Regulated by Nutritional Status, Obesity, or Leptin Levels in Rodents. Mediators Inflamm 2015, 620919 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun S. L., Lyu R. M., Chen Y. H., Chang J. K., Luo J. J. & Dun N. J. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 240, 155–162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H. M., Golozoubova V., Cannon B. & Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9, 203–209 (2009). [DOI] [PubMed] [Google Scholar]
- Lee P. et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab 19, 302–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme T. & Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol 300, R1–8 (2011). [DOI] [PubMed] [Google Scholar]
- Sivitz W. I., Fink B. D. & Donohoue P. A. Fasting and leptin modulate adipose and muscle uncoupling protein: divergent effects between messenger ribonucleic acid and protein expression. Endocrinology 140, 1511–1519 (1999). [DOI] [PubMed] [Google Scholar]
- Rothwell N. J. & Stock M. J. A role for insulin in the diet-induced thermogenesis of cafeteria-fed rats. Metabolism 30, 673–678 (1981). [DOI] [PubMed] [Google Scholar]
- Shibata H., Perusse F. & Bukowiecki L. J. The role of insulin in nonshivering thermogenesis. Can J Physiol Pharmacol 65, 152–158 (1987). [DOI] [PubMed] [Google Scholar]
- Gunawardana S. C. & Piston D. W. Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am J Physiol Endocrinol Metab 308, E1043–1055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusin I. et al. Chronic central leptin infusion enhances insulin-stimulated glucose metabolism and favors the expression of uncoupling proteins. Diabetes 47, 1014–1019 (1998). [DOI] [PubMed] [Google Scholar]
- Gesta S., Tseng Y. H. & Kahn C. R. Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 (2007). [DOI] [PubMed] [Google Scholar]
- Tran T. T., Yamamoto Y., Gesta S. & Kahn C. R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7, 410–420 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford K. I. et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64, 2002–2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Chen X., Chen Y. & Zhao Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int J Clin Exp Pathol 8, 6490–6497 (2015). [PMC free article] [PubMed] [Google Scholar]
- Catalano K. J., Stefanovski D. & Bergman R. N. Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes 59, 1416–1423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A. et al. Leptin administration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in mice. Int J Obes (Lond) 39, 397–407 (2015). [DOI] [PubMed] [Google Scholar]
- Stengel A., Hofmann T., Goebel-Stengel M., Elbelt U., Kobelt P. & Klapp B. F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity–correlation with body mass index. Peptides 39, 125–130 (2013). [DOI] [PubMed] [Google Scholar]
- Li L., Rampersad S., Wang X., Cheng X. & Qu S. Serum irisin concentrations were increased after transient continuous subcutaneous insulin infusion in type 2 diabetes mellitus patients. Diabetes Res Clin Pract 113, 44–47 (2016). [DOI] [PubMed] [Google Scholar]
- Yang Z., Chen X., Chen Y. & Zhao Q. PGC-1 mediates the regulation of metformin in muscle irisin expression and function. Am J Transl Res 7, 1850–1859 (2015). [PMC free article] [PubMed] [Google Scholar]
- Li D. J., Huang F., Lu W. J., Jiang G. J., Deng Y. P. & Shen F. M. Metformin promotes irisin release from murine skeletal muscle independently of AMP-activated protein kinase activation. Acta Physiol (Oxf ) 213, 711–721 (2015). [DOI] [PubMed] [Google Scholar]
- Li M. et al. Elevated circulating levels of irisin and the effect of metformin treatment in women with polycystic ovary syndrome. J Clin Endocrinol Metab 100, 1485–1493 (2015). [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Irisin and FNDC5 in retrospect: An exercise hormone or a transmembrane receptor? Adipocyte 2, 289–293 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]