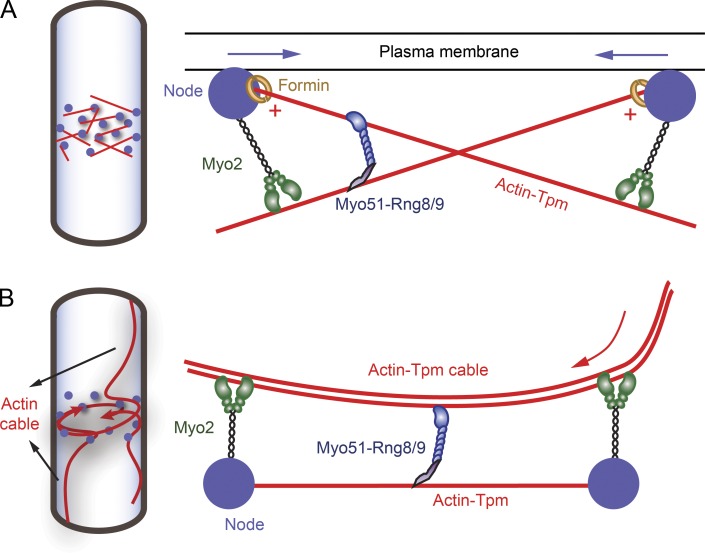
Figure 9.
Models illustrating how Myo51–Rng8/9 may function during fission yeast contractile ring assembly. (A) In the search, capture, pull and release (SCPR) model, actin filaments (red lines) were polymerized by formin (open yellow circles) in the precursor nodes (filled blue circles) that were accumulated equatorially at the early stage of cytokinesis and captured predominantly by Myo2 (green, a class II myosin) from adjacent nodes. Actin filaments are bound to acetylated fission yeast tropomyosin. Myo2 exerts force on actin-Tpm and moves toward the plus end (red plus symbols) of the filament (where the formin is bound), thus moving the nodes closer to each other (blue arrows indicate the movement of the nodes). Myo51–Rng8/9 anchors its tail on one actin-Tpm filament while sliding a nearby actin-Tpm filament, providing additional force and movement, presumably toward the plus end of actin-Tpm as well, resulting in efficient ring compaction. (B) In the cortical flow model, actin cables polymerized nonmedially are recruited into the contractile ring during ring assembly, in addition to the actin polymerized at the nodes (SCPR model). The actin cables are bound to and stabilized by fission yeast Tpm. The cable movement (indicated by the red arrow) into the ring and the subsequent compaction is driven by Myo2 and Myo51–Rng8/9. Myo51–Rng8/9 tail binds to an actin-Tpm filament or cable captured between the nodes and its motor domain assists the continuous transport of the actin cable into the contractile ring.