Abstract
Radical prostatectomy (RP) outcomes have been studied in White and Black non-Hispanic men qualifying for Epstein active surveillance criteria (EASC). Herein, we first analyzed such outcomes in White Hispanic men. We studied 70 men with nonpalpable Gleason score 3+3 = 6 (Grade Group [GG] 1) prostate cancer (PCa) with ≤2 positive cores on biopsy who underwent RP. In 18 men, prostate-specific antigen (PSA) density (PSAD) was >0.15 ng/mL/g. Three of these had insignificant and 15 had significant PCa. The remaining 52 men qualified for EASC. One patient had no PCa identified at RP. Nineteen (37%) had significant PCa defined by volume (n = 7), grade (n = 7), and volume and grade (n = 5). Nine cases were 3+4 = 7 (GG 2) (5/9 [56%] with pattern 4 <5%), 2 were 3+5 = 8 (GG 4), and 1 was 4+5 = 9 (GG 5). Patients with significant PCa more commonly had anterior dominant disease (11/19, 58%) versus patients with insignificant cancer (7/33, 21%) (P = 0.01). In 12 cases with higher grade at RP, the dominant tumor nodule was anterior in 6 (50%) and posterior in 6 (median volumes: 1.1 vs. 0.17 cm3, respectively; P = 0.01). PSA correlated poorly with tumor volume (r = 0.28, P = 0.049). Gland weight significantly correlated with PSA (r = 0.54, P < 0.001). While PSAD and PSA mass density correlated with tumor volume, only PSA mass density distinguished cases with significant disease (median, 0.008 vs. 0.012 μg/g; P = 0.03). In summary, a PSAD threshold of 0.15 works well in predicting significant tumor volume in Hispanic men. EASC appear to perform better in White Hispanic men than previously reported outcomes for Black non-Hispanic and worse than in White non-Hispanic men. Significant disease is often Gleason score 3+3 = 6 (GG 1) PCa >0.5 cm3. Significant PCa is either a larger-volume anterior disease that may be detected by multi-parametric magnetic resonance imaging-targeted biopsy or anterior sampling of the prostate or higher-grade smaller-volume posterior disease that in most cases should not pose immediate harm and may be detected by repeat template biopsies.
Keywords: prostate cancer, prostatectomy, Hispanic, very low risk, surveillance
Since 1994, when based on detailed clinicopathologic analysis Epstein et al1 described the most widely accepted active surveillance (AS) criteria for prostate cancer (PCa), the spectrum of disease has changed. In part, this is due to robust screening, which has resulted in a larger proportion of men contemporarily diagnosed with PCa who are candidates for AS.2 These AS criteria were adopted by The National Comprehensive Cancer Network (NCCN) and are widely accepted.3 Men defined as having very low-risk PCa should have a prostate-specific antigen (PSA) density (PSAD)≤0.15 ng/mL/g, unremarkable digital rectal examination (stage T1c), involvement of 2 or less biopsy cores with Gleason score 3+3 = 6 (Grade Group [GG] 1) disease, and 50% or less involvement of any single core.1 These criteria were developed from a cohort comprising >95% of White non-Hispanic men and 5 needle cores (median) per biopsy. This may not apply to more contemporary cohorts in the United States, with more ethnically heterogenous population and a minimum of 12 cores taken at the time of prostate biopsy.4,5 We previously investigated the performance of AS criteria in contemporary cohorts of White and Black non-Hispanic men and whether a higher number of positive cores may be allowed in a 12-core biopsy compared with the original Epstein active surveillance criteria (EASC).6,7 The predictive value of AS criteria has not been tested in Hispanic/Latino (later referred to as Hispanic) men. As the Hispanic population is rapidly increasing in the United States and already represents a high percentage of individuals in some states (http://www.cdc.gov/minorityhealth/; http://edr.state.fl.us/Content/population-demographics/data/Methodology_Projections_ARSH.pdf), we believe this is an increasingly important clinical challenge and have performed such a study.
MATERIALS AND METHODS
We studied 52 consecutive Hispanic men who underwent radical prostatectomy (RP) at the University of Miami Miller School of Medicine from 2004 to 2015 and qualified for original Epstein/NCCN AS criteria1 or their recent modification6 (Table 1). We also studied 18 men who had PSAD > 0.15 ng/mL/g and would have otherwise qualified for AS in order to test whether this threshold remained appropriate for Hispanic men to predict insignificant tumor volume.8 Data from 2004 to 2010 were obtained from a prospective urology database. Information for the patients operated more recently was extracted directly from the medical records by 2 authors (K.L. and F.M.C.). Patient ethnicity was verified using a set of criteria established by the North American Association of Central Cancer Registries (NAACCR) in the National Hispanic Identification Algorithm (NHIA).9 To establish ethnicity, these criteria weigh in surnames and race as variables by systematically classifying surnames as heavily, generally, moderately, occasionally, or rarely Hispanic based on the 1990 US Census Bureau Spanish Surname List. All last names that are categorized as “heavily” Hispanic were coded as Hispanic, and all others were coded as non-Hispanic.10,11 Race information was used by the algorithm to eliminate candidates with Spanish surnames who were identified as Asian, American Indian, Aleutian, Eskimo, Filipino, or Hawaiian. The NHIA algorithm was used in SAS 9.4 for Windows.
TABLE 1.
Original and Modified EASC for Nonpalpable (Stage T1c) PCa Detected by Template Biopsy
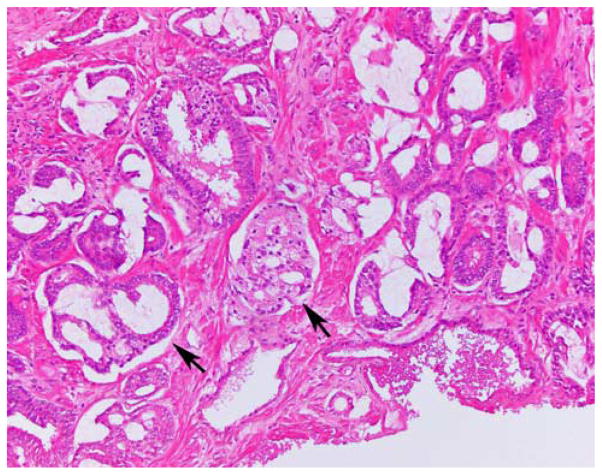
We selected men with a minimum of 10 cores on extended template biopsy to reflect a more contemporary practice and minimize heterogeneity of sampling.4,6,7,12 All prostate biopsies were reviewed at our institution before RP. All RP specimens were weighed, inked, fixed overnight in ambient formalin without injection, cut at 3 mm intervals from apex to base, and submitted as quadrants for histologic examination in regular size histologic cassettes. Basal portions of seminal vesicles (SVs) continuous with prostate were submitted for histologic analysis. Adipose tissue in pelvic lymph node dissection specimens was submitted in toto for histologic analysis after dissection of grossly visible lymph nodes.13 As our study included patients over a time period when International Society of Urological Pathology (ISUP) conducted 2 modifications of PCa grading,14–16 we re-reviewed biopsies for cases that were upgraded at RP and identified 1 case from 2004 that had occasional small cribriform glands graded as pattern 4 in current practice (Fig. 1). We excluded this case from analysis as it did not qualify for AS by biopsy Gleason score.
FIGURE 1.
Biopsy specimen interpreted in 2004 as Gleason score 3+3 = 6 (GG 1) disease and contemporarily graded as 3+4 = 7 (GG 2) due to the presence of small cribriform glands (arrows).
All histologic specimens were re-reviewed by 1 urologic pathologist (O.N.K.). Each tumor nodule was mapped on histologic slides and assigned an individual Gleason score (GG) on the basis of 2014 ISUP consensus and stage.15,16 Nodules located anterior to the prostatic urethra were considered anterior.7 Tertiary grade was restricted only to pattern 5 in 3+4 = 7 (GG 2) and 4+3 = 7 (GG 3) cases with the former constituting <5% of tumor nodules.16 Tumor nodules with predominant pattern 3 and minimal pattern 4 (< 5%) were assigned Gleason score 3+4 = 7 (GG 2) with recording the percentage of pattern 4.16 To determine tumor volume in each nodule, slides were photocopied in a background of 1 mm2 grid, and the amount of mm2 in each tumor nodule was manually counted. To convert square into cubic millimeters, the total number of mm2 was multiplied by 3 (thickness of prostate tissue sections) and 1.12 (fixation shrinkage factor).6,7,12 In cases with Gleason score 3+3 = 6 (GG 1) cancer at RP, disease was considered significant if the volume of the dominant tumor nodule was >0.5 cm3 or if extraprostatic extension was present. Any tumor nodule with Gleason score 3+4 = 7 (GG 2) and higher was considered significant PCa because of the grade. For cases with significant cancer at RP, a mis-classification category from 1 (least significant) to 4 (most significant) was assigned (Table 2). These categories were derived from a prior survey among PCa experts who classified cancer aggressiveness at RP on a scale of 1 to 4 with increasing severity based on tumor volume, Gleason score, and pathologic stage.6 We compared RP findings in Hispanic men with those in White and Black non-Hispanic men reported in a prior study.6 These 3 groups were comparable as they all satisfied preoperative EASC, both institutions followed similar RP histologic submission protocols, and the same pathologist reviewed cases in both studies.
TABLE 2.
Categorization of RP Cases Misclassified Preoperatively as Having Insignificant PCa
| Category 1 | Category 2 | Category 3 | Category 4 |
|---|---|---|---|
| GS 3+3 = 6 (GG 1), DTNV > 0.5 cm3, and OC or fEPE | GS 3+4 = 7 (GG 2) and OC | GS 3+4 = 7 (GG 2) with nfEPE | GS > 4+3 = 7 (GG 3) |
The definitions of misclassification categories are adopted from a prior PCa expert survey.6 Only misclassifications encountered in this study are included in the table. Tumor nodules with dominant Gleason pattern 3 and patter 4 <5% are classified as Gleason score 3+4 = 7 (GG 2) according to the 2014 International Society of Urologic Pathology recommendation.8
DTNV indicates dominant tumor nodule volume; fEPE, focal extraprostatic extension; GS, Gleason score; nfEPE, nonfocal extraprostatic extension; OC, organ confined.
Body mass index (BMI) was calculated as pre-operative patient weight in kilograms divided by the square of height in meters. BMI was categorized according to the World Health Organization classification into 4 tiers: underweight (BMI < 18.5), normal weight (BMI 18.5 to 24.99), overweight (BMI 25 to 29.99), and obese (BMI≥30). PSAD was calculated by dividing the preoperative serum PSA level by prostate weight without SVs.1,6,12,17 PSA mass (PSAM) was calculated by multiplying plasma volume (L) by PSA.18 Plasma volume was calculated by multiplying estimated body surface area (m2) by a 1.67 adjustment factor. Estimated body surface area was calculated by the following formula: (body weight, kg)0.425*(height, m)0.72*0.007184.18 PSA mass density (PSAMD) was calculated by dividing PSAM (μg) by prostate weight (g) without SV.17,19
Descriptive statistics were used to characterize the study cohort. Means were compared using unpaired t test when appropriate. Normality of the distribution of variables was assessed by the Anderson-Darling test. For variables in which the assumption for t test was not met (ie, approximately normally distributed variables), the Wilcoxon-Mann-Whitney rank sum test (U test) was used to compare means. Categorical outcomes were compared by the Fisher exact test. Strengths of associations were assessed by the Spearman rank correlation coefficient. Results were considered statistically significantly different with 2-tailed P-value <0.05. The study was approved by the Institutional Review Board of The University of Miami Miller School of Medicine.
RESULTS
A total of 466 Hispanic men underwent RP with total submission of prostate for histologic examination at our institution from 2004 to 2015. Seventy-six (16.3%) of them were diagnosed by extended (10 or more cores) biopsy because of PSA screening (T1c) and had Gleason score 6 (GG 1) PCa with ≤2 positive cores. Our study cohort was reduced to 70 cases because RP slides were not available for review in 6 cases (2 with PSAD > 0.15). Record review identified all cases as White Hispanic men. In 18 men, PSAD was >0.15 ng/mL/g. Three of them had insignificant PCa (2 had severe chronic inflammation) and 15 had significant PCa defined by volume of dominant tumor nodule >0.5 cm3 (n = 4), Gleason score 3+4 = 7 (GG 2) or higher (n = 1), and volume and grade (n = 10). The remaining 52 men qualified for either the original1 or modified6 EASC, and their clinical and RP findings are presented below.
Of the 45 (87%) men who underwent pelvic lymph node dissection, none had lymph node metastases. Nineteen patients (37%) had significant and 33 (63%) had insignificant disease at RP (Table 3). One patient had no PCa identified at RP following the appropriate examination protocol for such cases.20 Seventeen (33%) men had normal BMI, 24 (46%) were overweight, and 11 (21%) were obese. Patients with significant and insignificant cancer were not different by age, serum PSA level, BMI, PSAD, PSAM, number of cores involved, or highest percentage of core involvement by cancer (Table 3). PSAMD was the only measure that was significantly different between the 2 groups (P = 0.03).
TABLE 3.
Clinical and Radical Prostatectomy Findings in Hispanic Men Qualifying for EASC
| Study Groups
|
||||
|---|---|---|---|---|
| Parameter | General* (n = 52) | Insignificant PCa* (n = 33) | Significant PCa* (n = 19) | P |
| Age (y) | 58.2 (60.5, 43–70) | 59.0 (59.0, 43–70) | 58.7 (61, 43–69) | 0.9 |
| BMI | 27 (27, 18.6–35.4) | 27.3 (27, 18.6–35.4) | 26.3 (27, 20.7–31) | 0.3 |
| PSA (ng/mL) | 4.2 (4.3, 0.3–9.3) | 4.0 (3.8, 0.3–9.3) | 4.5 (4.7, 1.4–7.3) | 0.4 |
| PSAD (ng/mL/g)† | 0.08 (0.07, 0.02–0.15) | 0.08 (0.06, 0.02–0.15) | 0.1 (0.1, 0.03–0.15) | 0.07 |
| PSAM (μg) | 0.48 (0.49, 0.04–1.08) | 0.45 (0.46, 0.04–1.08) | 0.53 (0.53, 0.18–0.96) | 0.2 |
| PSAMD (μg/g) | 0.01 (0.009, 0.002–0.02) | 0.009 (0.008, 0.002–0.016) | 0.012 (0.012, 0.004–0.02) | 0.03 |
| # of positive cores | ||||
| 1 | 28 | 18 | 10 | 1.0 |
| 2 | 24 | 15 | 9 | |
| Max % of core involvement | 13 (10, 2–50) | 13 (10, 2–50) | 14 (15, 2–30) | 0.7 |
| Prostate weight (g)† | 51.2 (45, 20–158) | 62.6 (41, 20–158) | 48.9 (45, 31–97) | 0.8 |
| Dominant tumor nodule volume (cm3)†‡ | 0.4 (0.12, 0.004–2.69) | 0.13 (0.07, 0.004–0.5) | 0.87 (0.71, 0.09–2.69) | < 0.001 |
| Total tumor volume (cm3)†‡ | 0.49 (0.28, 0.004–2.98) | 0.17 (0.1, 0.004–0.61) | 1.0 (0.97, 0.14–2.98) | < 0.001 |
| Anterior dominant tumor nodule (n [%]) | 18/53 (34) | 7/33 (21) | 11/19 (58) | 0.01 |
| Stage‡ (n [%]) | ||||
| pT2 | 43 (82) | 30 (94) | 13 (65) | 0.01 |
| pT2+ | 5 (10) | 2 (6) | 3 (15) | |
| pT3 | 4 (8) | 0 | 4 (20) | |
Data in mean (median, range).
Wilcoxon-Mann-Whitney rank sum test (U test).
Biopsy-proven case with no cancer identified at RP is not included in statistical calculation.
Two (6%) cases with insignificant disease had positive surgical margin in the area of intraprostatic incision (pT2+ stage). Four (21%) cases with significant disease had positive margins (3 pT2+ and 1 pT3 stage). Three cases with significant disease had extraprostatic extension (1 focal and 2 nonfocal; pT3a stage), and 1 case had SV invasion (pT3b stage).
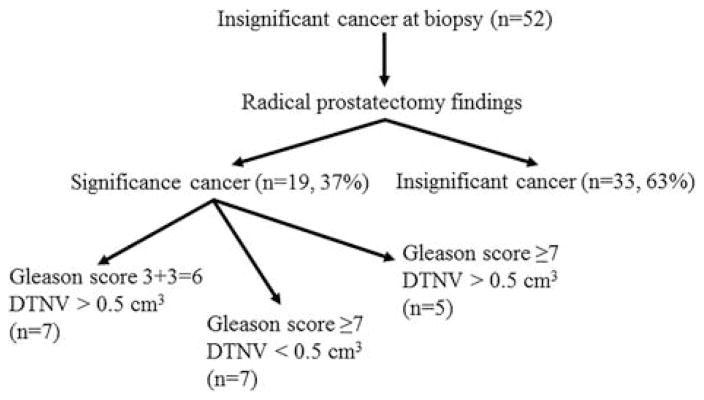
Nineteen cases with significant cancer at RP were determined by volume of dominant tumor nodule >0.5cm3 (n=7), Gleason score 3+4=7 (GG 2) or higher (n= 7), and volume and grade (n=5) (Fig. 2). Nine cases were Gleason score 3+4=7 (GG 2) (5 [56%] cases had percentage pattern 4 <5% in the dominant tumor nodule), 2 were 3+5=8 (GG 4), and 1 was 4+5=9 (GG 5). Although 1 case with Gleason score 3+3=6 (GG 1) dominant tumor nodule of 1.65cm3 had focal extraprostatic extension, none of the cases with significant disease with Gleason score 3+3=6 (GG 1) was defined as such solely by extraprostatic extension. The misclassification categories of significant PCa at RP were: category 1: 7 cases, category 2: 7 cases, category 3: 2 cases, and category 4: 3 cases. Because of a small number of cases in categories 3 and 4, we combined them into a single group and compared the incidence of different categories of aggressiveness with our previously published data (Table 4).
FIGURE 2.
RP findings in Hispanic men qualifying for AS. DTNV indicates dominant tumor nodule volume.
TABLE 4.
Incidence of Overall and Individual Misclassification Categories in Different Ethnicities Qualifying for EASC
| Ethnicity | Overall | Category 1 | Category 2 | Category 3–4 |
|---|---|---|---|---|
| White non-Hispanic6* (n [%]) | 50/185 (27) | 9 (18) | 37 (74) | 4 (8) |
| Black non-Hispanic6* (n [%]) | 32/62 (51.6) | 4 (12.5) | 25 (78) | 3 (9.5) |
| White Hispanic (n [%]) | 19/52 (36.5) | 7 (37) | 7 (37) | 5 (26) |
| P | 0.002 | 0.02 |
Data from a prior publication.6 All prostatectomies in the current and prior publication were reviewed by the same urologic pathologist (O.N.K.).
Overall, cases misclassified by biopsy as insignificant disease were more likely to have anterior dominant tumor nodules. Seventy-one percent (5/7) of Gleason score 3+3 = 6 (GG 1) cases qualifying for significant disease by volume of dominant tumor nodule had anterior dominant disease averaging 1.36 cm3 (median, 1.08; range, 0.8 to 2.43). Two cases with significant size posterior dominant tumor nodules had corresponding tumor volumes of 0.54 and 0.71 cm3.
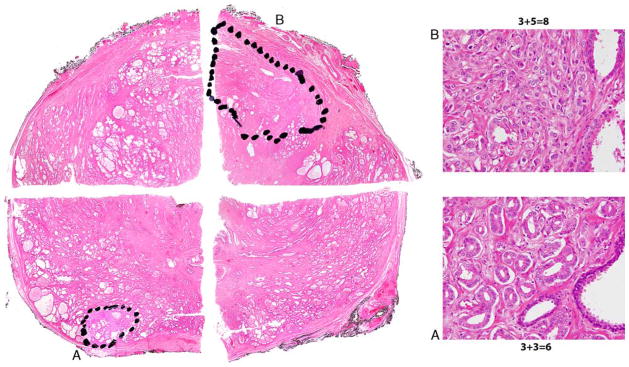
Among 12 cases with Gleason upgrade at RP, the dominant tumor nodule was anterior in 6 (50%) cases with an average dominant tumor nodule volume of 1.18 cm3 (median, 1.1; range, 0.32 to 2.69). Three cases with the most significant upgrade (Gleason score 3+5 = 8 [GG 4] and 4+5 = 9 [GG 5]) had anterior dominant tumor nodules. Five of 6 cases with anterior dominant higher-grade disease had secondary Gleason score 3+3 = 6 (GG 1) tumor nodules in the posterior prostate (Fig. 3). In all these cases, the dominant tumor nodule defined by grade was also the largest tumor nodule. The remaining case with 1 anterior dominant Gleason score 3+4 = 7 (GG 2) tumor nodule had <5% of pattern 4. In 6 cases with upgraded posterior dominant disease, the average dominant tumor nodule volume was 0.23 cm3 (median, 0.17; range, 0.09 to 0.45). The difference between the average volume of dominant tumor nodule in cases with anterior and posterior dominant disease was significant (P = 0.01; U test). Three cases had a single posterior tumor nodule with average volume of 0.35 cm3 (median, 0.39; range, 0.22 to 0.45). In 3 cases with >1 tumor nodule, the average volume of dominant posterior tumor nodule defined by grade was 0.11 cm3 (median, 0.1; range, 0.09 to 0.13), and it was not the largest tumor nodule in 2 of these cases. All 6 upgraded cases with posterior dominant tumor nodules were Gleason score 3+4 = 7 (GG 2). Four (67%) of these had <5% of pattern 4.
FIGURE 3.
Pseudo whole mount demonstrating biopsy sampled Gleason score 3+3 = 6 (GG 1) tumor nodule in right posterolateral mid (A) and anterior dominant unsampled tumor nodule with Gleason score 3+5 = 8 (GG 4) disease in left anterior mid (B).
Total tumor volume had the best correlation with PSAMD and only showed borderline significant correlation with PSA (Table 5). Dominant tumor nodule volume did not have a significant correlation with either PSA or PSAM. Gland weight strongly correlated with PSA and PSAM. PSAMD correlations with dominant tumor nodule and total tumor volume were slightly better than PSAD.
TABLE 5.
Correlation of Dominant Tumor Nodule Volume, Total Tumor Volume, and Gland Weight With PSA and Its Derivatives
|
P
|
||||
|---|---|---|---|---|
| PSA | PSAD | PSAM | PSAMD | |
| DTNV | 0.25 (0.07) | 0.41 (0.003) | 0.27 (0.06) | 0.43 (0.002) |
| TTV | 0.28 (0.049) | 0.37 (0.007) | 0.31 (0.03) | 0.41 (0.003) |
| Gland weight | 0.54 (< 0.001) | 0.001 (1.0) | 0.56 (< 0.001) | 0.03 (0.8) |
Spearman rank correlation.
DTNV indicates dominant tumor nodule volume; TTV, total tumor volume.
DISCUSSION
The most widely used PCa biopsy AS criteria were developed by Epstein and colleagues in 1994.1,3 Qualifying patients were men with nonpalpable disease (T1c) with PSAD≤0.15 ng/mL/g, up to 2 positive cores with Gleason score ≤6 (GG 1) cancer, and ≤50% of any individual core involvement by cancer. Since then, there have been significant changes in clinical practice and pathologic understanding of PCa. From a median of 5 cores in 1994, the usual number of cores sampled at first biopsy has increased to 12 including a standard sextant biopsy and lateral sextant samples.4,5 Epstein et al1 originally reported that approximately 16% of men diagnosed due to PSA screening (T1c) were amenable for AS. In contemporary practice, this percentage approaches 40% using the same criteria.2 Although >95% of men in the work by Epstein and colleagues were White non-Hispanic men, the ethnicity of contemporary men diagnosed with PCa is significantly more diverse.2,6,7 Hispanics are the largest and the most rapidly growing ethnic minority in the United States, and it is predicted that Hispanics will comprise a third of the population by 2060 (http://www.cdc.gov/minorityhealth/populations/REMP/hispanic.html; http://edr.state.fl.us/Content/population-demographics/data/Methodology_Projections_ARSH.pdf). To date, there has been no detailed clinicopathologic analysis of RP findings in Hispanic men qualifying for AS. We have conducted a comprehensive analysis of clinical presentation and pathologic outcomes in a cohort composed of White Hispanic men who underwent RP but qualified for AS.
PCa is the most prevalent visceral malignancy in Hispanic men.11 Approximately 13,000 (22% of all cancers) cases were diagnosed in Hispanic men in 2015 with estimated PCa-related deaths of 1800 (9% of all deaths).11 This shows that similarly to White non-Hispanic men, PCa in the Hispanic population also has a rather indolent course. However, compared with White non-Hispanic men, where a higher proportion of newly diagnosed men were candidates for AS, only 11.2% (52/466) of Hispanic men in our study qualified for EASC. We cannot fully explain this low qualifying percentage, but in part it may be related to underscreening in Hispanic men,11 as well as other individual, cultural, and societal barriers interfering with access to PCa care in Hispanic men.21
Of the Hispanic men predicted to have insignificant cancer by EASC, 63% had insignificant disease at RP. In the 1994 Epstein et al1 study, the negative predictive value was 66% in a predominantly White non-Hispanic population, meaning that when the preoperative model predicted insignificant cancer at RP, 66% truly had insignificant cancer. In a recent work studying a contemporary cohort of men with extended prostate biopsy, the negative predictive value was 70.6% and 74.1% in White non-Hispanic and 45.9% and 48.4% in Black non-Hispanic men by the original and modified EASC, respectively.6
We studied a historical cohort of Hispanic men who underwent template extended prostate biopsies. A PSAD threshold of 0.15 ng/mL/g worked well in T1c Hispanic men with ≤2 positive cores for identifying men who were appropriate candidates for AS. Fourteen (78%) of 18 men with PSAD > 0.15 ng/mL/g had dominant tumor nodule volume >0.5 cm3. Two (11%) men with insignificant disease had marked inflammation likely leading to an elevated PSA that may have disqualified them from AS.22
Although there is increasing utilization of multi-parametric magnetic resonance imaging (MRI) to direct a prostate biopsy to radiologically suspicious areas, the NCCN recommended a template 12-core biopsy in the latest release of PCa early detection guidelines (December 2015).5 Anteriorly directed biopsies sampling bilateral transitional zones were not recommended during routine biopsy. Multiparametric MRI was not recommended for the first transrectal ultrasound-guided prostate biopsy, but should be considered before repeat biopsy in patients with a high clinical suspicion of PCa. Thus, most patients enrolled in AS will be followed up according to findings from extended template (12-core) prostate biopsy. At the University of Miami, patients electing AS after the original diagnostic template prostate biopsy undergo multi-parametric MRI and targeted prostate biopsy if lesions suspicious for cancer are detected (Miami Active Selection for Treatment [MAST] trial).
Compared with prior studies of men qualifying for AS with pathology review,1,6,7 this study was conducted according to the most contemporary standards of PCa pathology. In this respect, re-review of biopsies of cases upgraded at RP in our cohort identified and excluded 1 patient whose biopsy was correctly graded in 2004 as Gleason score 3+3 = 6 (GG 1) disease. This patient had occasional small cribriform glands at biopsy that were considered pattern 3 at that time.14 However, it was shown that even a minor percentage of small cribriform structures may lead to regional lymph node metastasis, and an appropriate pattern of these is 4.23 Another change according to contemporary reporting that is important in comparing the current results with prior publications is that 56% (5/9) of cases with Gleason score 3+4 = 7 (GG 2) dominant tumor nodules had <5% of pattern 4 that would have been recorded as tertiary pattern in the past.14–16 In studies that use data from urology or cancer registry databases, these cases may be recorded incorrectly, as tertiary pattern is often not accounted for.24 In the PCa expert survey preceding the 2014 ISUP meeting, participants considered Gleason score 3+3 = 6 (GG 1) with tertiary pattern 4 and 3+4 = 7 (GG 2) as category 2 misclassification.6 Using misclassification categories, we demonstrated that RP outcomes in Hispanic men showed a statistically significant difference from White and Black non-Hispanic men not only by frequency of incorrectly predicted insignificant disease, but also the aggressiveness of misclassified cases. Whereas in White and Black non-Hispanic men 80% to 90% of upgrades were defined by grade (Category 2 to 4), in Hispanic men 37% of significant cases were determined by larger-volume Gleason score 6 (GG 1) disease (Category 1)—cancer that potentially imparts less risk to the patient than higher misclassification categories. These cases have the potential to locally invade yet lack the ability to have lymph node metastases. Two works cumulatively studying 14,572 RPs with reported Gleason score 6 (GG 1) demonstrated that all cases recorded as metastatic to regional lymph nodes either had incorrect record in report/database or had higher grade on re-review.23,25 A recent study suggested increasing the limit of insignificant Gleason score 3+3 = 6 (GG 1) PCa to 2.5 cm3.26 However, this study preselected only organ-confined cases (pT2 stage) rather than all consecutive Gleason score 6 (GG 1) cases. In this cohort and prior studies with consecutive cases,6 we have identified extraprostatic extension in cases with lower tumor volumes and thus adhered in this study to a historically accepted threshold of insignificant disease as ≤0.5 cm3 dominant tumor nodule.1,6,7
Cases with significant disease were either larger-volume anterior dominant tumor nodules that may be sampled by anterior prostate biopsy or MRI-targeted biopsy on repeat/confirming AS biopsies or higher-grade smaller-volume posterior tumor nodules that in most cases would unlikely be of immediate danger to the patient even if not detected on the first repeat biopsy.21,27 These smaller tumor nodules not sampled by original diagnostic biopsy may later explain grade-related progression.26 Our RP findings in Hispanic men support recent conclusions from the Hopkins AS cohort (1298 White and Black non-Hispanic men) that individuals with favorable-risk PCa should be encouraged to consider AS.28
Although there were no statistically significant differences in PSA and PSAD in dichotomized analysis between men with significant and insignificant disease, patients with PSAD >0.15 had worse RP outcome, and PSAD correlated strongly with tumor volume. We believe the former lack of statistical significance may be explained by a relatively low number of enrolled individuals. PSA did not correlate with dominant tumor nodule volume and correlated only marginally with total tumor volume. Prostate weight had a major influence on PSA, as is often seen in generally low-volume disease in contemporary PCa detected by screening.12 The only measure that significantly distinguished cases with significant PCa was PSAMD. PSAMD also had the best correlation with tumor volume similar to our prior observations in White and Black non-Hispanic men.19 PSAMD accounts for BMI-related PSA hemodilution and thus is a PSA derivative that is reproducible and performs equally well in men with normal and excessive body weight. It is, however, in its early stage of development and lacks validated reference points. In view of its more complex formula, it may require some facilitating tools such as an online calculator using PSA, patient’s height and weight, and radiologic prostate volume.
A multiethnic population with often indistinct separation between different ethnicities distinguishes the United States. We studied how the Surveillance, Epidemiology, and End Results (SEER) Program assigns race/ethnicity on the basis of state cancer registries, which collect data on race and ethnicity from various data sources, including hospital records, medical records, pathology reports, hospital discharge data, and death certificates. SEER assigns Hispanic status through the same NHIA algorithm that was used for our patient cohort to determine Hispanic/Latino ethnicity.10,11 Thus, we believe our approach is the best-standardized methodology to select the study cohort matching our goals. Another point to be considered, often not mentioned in race/ethnicity-related studies, is that a common Hispanic denominator includes a diverse population. It is well reflected in the US Census Bureau and the US Office of Management and Budget definition of Hispanic ethnic classification as “a person of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin regardless of race” (http://edr.state.fl.us/Content/population-demographics/data/Methodology_Projections_ARSH.pdf). According to the US Census Bureau and Centers for Disease Control and Prevention, 63% and 64%, respectively, of the US Hispanic population are Mexican by origin (http://www.cdc.gov/minorityhealth/populations/REMP/hispanic.html; http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf). The Census data highlight Florida as the state with the third largest Hispanic population (8.4% of total US Hispanic population) after California (27.8%) and Texas (18.7%) (http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf). The Florida Hispanic origin according to the 2010 Census survey was mostly from Cuba (28%) and Puerto Rico (20%) with all other origins accounting for significantly smaller percentages. The high contribution from these regions of origin may explain the White Hispanic population in our study.
In summary, EASC perform well in White Hispanic men whose RP outcomes fall somewhere between previously reported results in White and Black non-Hispanic men. A PSAD threshold of 0.15 appears to be appropriate for AS. Approximately a third of significant tumors are Gleason score 6 (GG 1) cancers with tumor volume >0.5 cm3 that is potentially less dangerous compared with Gleason score upgrading that is more often seen in White and Black non-Hispanic men qualifying for AS. Significant cancers either have larger anterior dominant tumor nodules that may be identified by multiparametric MRI-targeted biopsy or anterior sampling of the prostate or smaller-volume posterior tumor nodules that in most cases should not pose immediate harm to the patient and may be detected by repeat template biopsies.
Acknowledgments
The authors thank Paula S. Espinal and Melinda M. Boone for help with the specimen handling at the University of Miami and acquisition of outside specimens, correspondingly. This study was supported by the National Institutes of Health (NIH) awards R01CA189295 (A. Pollack), R01CA190105 (R. Gillies/A. Pollack). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 2.Overholser S, Nielsen M, Torkko K, et al. Active surveillance is an appropriate management strategy for a proportion of men diagnosed with prostate cancer by prostate specific antigen testing. J Urol. 2015;194:680–684. doi: 10.1016/j.juro.2015.01.089. [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12:686–718. doi: 10.6004/jnccn.2014.0072. [DOI] [PubMed] [Google Scholar]
- 4.Kryvenko ON, Diaz M, Meier FA, et al. Findings in 12-core transrectal ultrasound-guided prostate needle biopsy that predict more advanced cancer at prostatectomy: analysis of 388 biopsy-prostatectomy pairs. Am J Clin Pathol. 2012;137:739–746. doi: 10.1309/AJCPWIZ9X2DMBEBM. [DOI] [PubMed] [Google Scholar]
- 5.Carroll PR, Parsons JK, Andriole G, et al. Prostate cancer early detection, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1534–1561. doi: 10.6004/jnccn.2015.0181. [DOI] [PubMed] [Google Scholar]
- 6.Kryvenko ON, Carter HB, Trock BJ, et al. Biopsy criteria for determining appropriateness for active surveillance in the modern era. Urology. 2014;83:869–874. doi: 10.1016/j.urology.2013.12.054. [DOI] [PubMed] [Google Scholar]
- 7.Sundi D, Kryvenko ON, Carter HB, et al. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol. 2014;191:60–67. doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kryvenko ON, Balise R, Prakash NS, et al. African-American men with Gleason Score 3+3 = 6 prostate cancer produce less prostate specific antigen than Caucasian men: a potential impact on active surveillance. J Urol. 2016;195:301–306. doi: 10.1016/j.juro.2015.08.089. [DOI] [PubMed] [Google Scholar]
- 9.NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1] [Accessed 20 April, 2016];2011 Available. at: http://www.naaccr.org/LinkClick.aspx?fileticket=6E20OT41TcA%3D.
- 10.Iqbal J, Ginsburg O, Rochon PA, et al. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313:165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin. 2015;65:457–480. doi: 10.3322/caac.21314. [DOI] [PubMed] [Google Scholar]
- 12.Swanson GP, Epstein JI, Ha CS, et al. Pathological characteristics of low risk prostate cancer based on totally embedded prostatectomy specimens. Prostate. 2015;75:424–429. doi: 10.1002/pros.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon JY, Kryvenko ON, Ghani KR, et al. Characteristics of pelvic lymph node metastases in prostatic adenocarcinoma: a study of 83 cases. Int J Surg Pathol. 2012;20:449–454. doi: 10.1177/1066896912445921. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 15.Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2015;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 16.Kryvenko ON, Epstein JI. Prostate cancer grading: a decade after the 2005 modifed Gleason grading system. Arch Pathol Lab Med. 2015 doi: 10.5858/arpa.2015-0487-SA. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Tjionas GA, Epstein JI, Williamson SR, et al. Average weight of seminal vesicles: an adjustment factor for radical prostatectomy specimens weighed with seminal vesicles. Int J Surg Pathol. 2015;23:617–622. doi: 10.1177/1066896915600519. [DOI] [PubMed] [Google Scholar]
- 18.Kryvenko ON, Epstein JI, Meier FA, et al. Correlation of high body mass index with more advanced localized prostate cancer at radical prostatectomy is not reflected in PSA level and PSA density but is seen in PSA mass. Am J Clin Pathol. 2015;144:271–277. doi: 10.1309/AJCPQL9MKQ6VDDWL. [DOI] [PubMed] [Google Scholar]
- 19.Kryvenko ON, Mireya D, Matoso A, et al. PSA mass density—a measure predicting prostate cancer volume and accounting for overweight and obesity related prostate-specific antigen hemodilution. Urology. 2016;90:141–147. doi: 10.1016/j.urology.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Duffield AS, Epstein JI. Detection of cancer in radical prostatectomy specimens with no residual carcinoma in the initial review of slides. Am J Surg Pathol. 2009;33:120–125. doi: 10.1097/PAS.0b013e318185723e. [DOI] [PubMed] [Google Scholar]
- 21.Dianat SS, Carter HB, Pienta KJ, et al. Magnetic resonance-invisible versus magnetic resonance-visible prostate cancer in active surveillance: a preliminary report on disease outcomes. Urology. 2015;85:147–153. doi: 10.1016/j.urology.2014.06.085. [DOI] [PubMed] [Google Scholar]
- 22.Kryvenko ON, Jankowski M, Chitale DA, et al. Inflammation and preneoplastic lesions in benign prostate as risk factors for prostate cancer. Mod Pathol. 2012;25:1023–1032. doi: 10.1038/modpathol.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross HM, Kryvenko ON, Cowan JE, et al. Do adenocarcinomas of the prostate with Gleason score (GS) </ = 6 have the potential to metastasize to lymph nodes? Am J Surg Pathol. 2012;36:1346–1352. doi: 10.1097/PAS.0b013e3182556dcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kryvenko ON, Epstein JI. Re: Nationwide prevalence of lymph node metastases in Gleason score 3+3 = 6 prostate cancer. Pathology. 2015;47:394. doi: 10.1097/PAT.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 25.Kweldam CF, Wildhagen MF, Bangma CH, et al. Disease-specific death and metastasis do not occur in patients with Gleason score </ = 6 at radical prostatectomy. BJU Int. 2015;116:230–235. doi: 10.1111/bju.12879. [DOI] [PubMed] [Google Scholar]
- 26.Wong LM, Alibhai SM, Trottier G, et al. A negative confirmatory biopsy among men on active surveillance for prostate cancer does not protect them from histologic grade progression. Eur Urol. 2014;66:406–413. doi: 10.1016/j.eururo.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 27.Kryvenko ON, Gupta NS, Virani N, et al. Gleason score 7 adenocarcinoma of the prostate with lymph node metastases: analysis of 184 radical prostatectomy specimens. Arch Pathol Lab Med. 2013;137:610–617. doi: 10.5858/arpa.2012-0128-OA. [DOI] [PubMed] [Google Scholar]
- 28.Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol. 2015;33:3379–3385. doi: 10.1200/JCO.2015.62.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]