Figure 5.
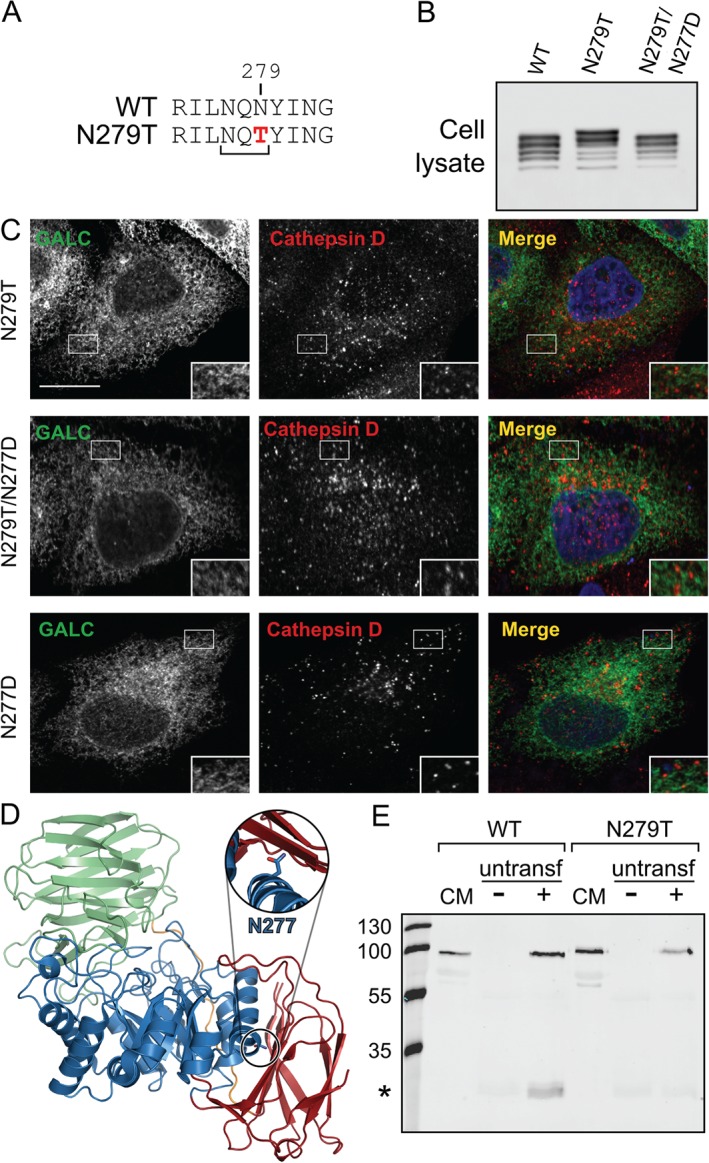
N279T is a hyperglycosylation mutant with defective trafficking. A) Protein sequence of WT and N279T GALC illustrating the introduction of a new consensus glycosylation site (NXT). B) HEK293T cells transiently transfected with WT GALC, Krabbe mutation N279T or double mutant N279T/N277D were lysed with 1% SDS, separated by SDS‐PAGE and immunoblotted. Total GALC expression was analysed using western blot against the FLAG epitope tag. C) HeLa cells were transiently transfected with Krabbe mutation N279T, the double mutation N279T/N277D or N277D alone, plated and fixed on glass coverslips and immunostained for GALC (green) and the lysosomal marker cathepsin D (red). Scale bar: 10 µm. D) The newly glycosylated residue, N277, is highlighted on the structure of GALC (PDB ID: 3ZR5). The structure is coloured according to domain (TIM barrel in blue, β‐sandwich in red and lectin domain in green). Zoomed view (inset) shows the N277 sidechain as sticks. E) Conditioned media from either untransfected HEK293T cells (−) or cells transfected with WT or Krabbe mutation N279T (+) was harvested after 72 h and applied to untransfected HEK293T cells for 48 h. Treated cells were subjected to immunoprecipitation using the GALC monoclonal antibody mAb2. GALC was detected using western blot with a polyclonal rabbit GALC antibody.
