Abstract
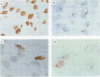
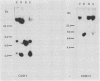
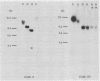
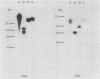
It was postulated that non-isotopic in situ hybridisation (NISH) signal types 1-3 for human papillomavirus in cervical biopsy specimens represent episomal or integrated virus. The aim of this study was to validate this hypothesis by independent molecular techniques. Fresh cervical intraepithelial neoplasia (CIN) and squamous cell cancer (SCC) tissue were examined for NISH signal pattern by hybridising with digoxigenin labelled HPV 16. DNA was extracted from the same samples and analysed by restriction endonuclease digestion and Southern blotting to determine the physical state of the viral genome. Six CIN biopsy specimens showed a type 1 NISH signal for HPV 16. On Southern analysis these biopsy specimens contained only episomal HPV 16. Three SCC with a type 2 NISH signal contained integrated HPV 16 by Southern analysis. Two specimens, a CIN 3 and an SCC with a type 3 NISH signal for HPV 16, showed the presence of both episomal and integrated HPV 16 with conventional Southern analysis and two dimensional gel electrophoresis. These results show that episomal HPV can be reliably determined by NISH type 1 signal, integrated HPV by type 2, and a combination of both episomal and integrated HPV, by a type 3 signal in archival paraffin wax embedded cervical biopsy specimens. This will add another variable to the epidemiological studies of HPV infection. In particular, it will now allow retrospective studies to be done to define the role of episomal and integrated HPV in the evolution of cervical intraepithelial neoplasia and other cervical disease associated with this virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Phelps W. C., Lindgren V., Braun M. J., Gonda M. A., Howley P. M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K. B., Cheung W. F., Liew L. N., Lee H. H., Han S. H. Presence of catenated human papillomavirus type 16 episomes in a cervical carcinoma cell line. J Virol. 1989 Feb;63(2):782–789. doi: 10.1128/jvi.63.2.782-789.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K., Herrington C. S., Graham A. K., Evans M. F., McGee J. O. In situ evidence for HPV 16, 18, 33 integration in cervical squamous cell cancer in Britain and South Africa. J Clin Pathol. 1991 May;44(5):406–409. doi: 10.1136/jcp.44.5.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K., Herrington C. S., Graham A. K., Evans M. F., McGee J. O. In situ human papillomavirus (HPV) genotyping of cervical intraepithelial neoplasia in South African and British patients: evidence for putative HPV integration in vivo. J Clin Pathol. 1991 May;44(5):400–405. doi: 10.1136/jcp.44.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen A. P., Reid R., Campion M., Lörincz A. T. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991 Feb;65(2):606–612. doi: 10.1128/jvi.65.2.606-612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Luca D., Pilotti S., Stefanon B., Rotola A., Monini P., Tognon M., De Palo G., Rilke F., Cassai E. Human papillomavirus type 16 DNA in genital tumours: a pathological and molecular analysis. J Gen Virol. 1986 Mar;67(Pt 3):583–589. doi: 10.1099/0022-1317-67-3-583. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Kleinheinz A., Hotz M., Gissmann L. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J Gen Virol. 1985 Jul;66(Pt 7):1515–1522. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- Fukushima M., Yamakawa Y., Shimano S., Hashimoto M., Sawada Y., Fujinaga K. The physical state of human papillomavirus 16 DNA in cervical carcinoma and cervical intraepithelial neoplasia. Cancer. 1990 Nov 15;66(10):2155–2161. doi: 10.1002/1097-0142(19901115)66:10<2155::aid-cncr2820661019>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Herrington C. S., Burns J., Graham A. K., Evans M., McGee J. O. Interphase cytogenetics using biotin and digoxigenin labelled probes I: relative sensitivity of both reporter molecules for detection of HPV16 in CaSki cells. J Clin Pathol. 1989 Jun;42(6):592–600. doi: 10.1136/jcp.42.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Kataja V., Syrjänen K., Mäntyjärvi R., Väyrynen M., Syrjänen S., Saarikoski S., Parkkinen S., Yliskoski M., Salonen J. T., Castren O. Prospective follow-up of cervical HPV infections: life table analysis of histopathological, cytological and colposcopic data. Eur J Epidemiol. 1989 Mar;5(1):1–7. doi: 10.1007/BF00145037. [DOI] [PubMed] [Google Scholar]
- Kataja V., Syrjänen K., Syrjänen S., Mäntyjärvi R., Yliskoski M., Saarikoski S., Salonen J. T. Prospective follow-up of genital HPV infections: survival analysis of the HPV typing data. Eur J Epidemiol. 1990 Mar;6(1):9–14. doi: 10.1007/BF00155542. [DOI] [PubMed] [Google Scholar]
- Lehn H., Villa L. L., Marziona F., Hilgarth M., Hillemans H. G., Sauer G. Physical state and biological activity of human papillomavirus genomes in precancerous lesions of the female genital tract. J Gen Virol. 1988 Jan;69(Pt 1):187–196. doi: 10.1099/0022-1317-69-1-187. [DOI] [PubMed] [Google Scholar]
- Matlashewski G. The cell biology of human papillomavirus transformed cells. Anticancer Res. 1989 Sep-Oct;9(5):1447–1456. [PubMed] [Google Scholar]
- Meanwell C. A., Cox M. F., Blackledge G., Maitland N. J. HPV 16 DNA in normal and malignant cervical epithelium: implications for the aetiology and behaviour of cervical neoplasia. Lancet. 1987 Mar 28;1(8535):703–707. doi: 10.1016/s0140-6736(87)90353-9. [DOI] [PubMed] [Google Scholar]
- Mincheva A., Gissmann L., zur Hausen H. Chromosomal integration sites of human papillomavirus DNA in three cervical cancer cell lines mapped by in situ hybridization. Med Microbiol Immunol. 1987;176(5):245–256. doi: 10.1007/BF00190531. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Pater A. Human papillomavirus types 16 and 18 sequences in carcinoma cell lines of the cervix. Virology. 1985 Sep;145(2):313–318. doi: 10.1016/0042-6822(85)90164-3. [DOI] [PubMed] [Google Scholar]
- Popescu N. C., DiPaolo J. A. Integration of human papillomavirus 16 DNA and genomic rearrangements in immortalized human keratinocyte lines. Cancer Res. 1990 Feb 15;50(4):1316–1323. [PubMed] [Google Scholar]
- Shirasawa H., Tomita Y., Kubota K., Kasai T., Sekiya S., Takamizawa H., Simizu B. Detection of human papillomavirus type 16 DNA and evidence for integration into the cell DNA in cervical dysplasia. J Gen Virol. 1986 Sep;67(Pt 9):2011–2015. doi: 10.1099/0022-1317-67-9-2011. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Syrjänen S., Partanen P., Mäntyjärvi R., Syrjänen K. Sensitivity of in situ hybridization techniques using biotin- and 35S-labeled human papillomavirus (HPV) DNA probes. J Virol Methods. 1988 Mar-Apr;19(3-4):225–238. doi: 10.1016/0166-0934(88)90017-1. [DOI] [PubMed] [Google Scholar]
- Yee C., Krishnan-Hewlett I., Baker C. C., Schlegel R., Howley P. M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol. 1985 Jun;119(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- Yee C., Krishnan-Hewlett I., Baker C. C., Schlegel R., Howley P. M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol. 1985 Jun;119(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- de Villiers E. M. Heterogeneity of the human papillomavirus group. J Virol. 1989 Nov;63(11):4898–4903. doi: 10.1128/jvi.63.11.4898-4903.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]