Summary
The exported protein fraction of an organism may reflect its life strategy and, ultimately, the way it is perceived by the outside world. Bioinformatic prediction of the exported pan‐proteome of P rochlorococcus and S ynechococcus lineages demonstrated that (i) this fraction of the encoded proteome had a much higher incidence of lineage‐specific proteins than the cytosolic fraction (57% and 73% homologue incidence respectively) and (ii) exported proteins are largely uncharacterized to date (54%) compared with proteins from the cytosolic fraction (35%). This suggests that the genomic and functional diversity of these organisms lies largely in the diverse pool of novel functions these organisms export to/through their membranes playing a key role in community diversification, e.g. for niche partitioning or evading predation. Experimental exoproteome analysis of marine S ynechococcus showed transport systems for inorganic nutrients, an interesting array of strain‐specific exoproteins involved in mutualistic or hostile interactions (i.e. hemolysins, pilins, adhesins), and exoenzymes with a potential mixotrophic goal (i.e. exoproteases and chitinases). We also show how these organisms can remodel their exoproteome, i.e. by increasing the repertoire of interaction proteins when grown in the presence of a heterotroph or decrease exposure to prey when grown in the dark. Finally, our data indicate that heterotrophic bacteria can feed on the exoproteome of S ynechococcus.
Introduction
The exoproteome is the protein fraction found in the extracellular proximity of one or several organisms. It comprises actively exported proteins and those that are released by cell lysis or leakage. For example, the exoproteomes of pure cultures are those proteins that can be detected after purification from the culture medium once cells have been removed (Armengaud et al., 2012). These proteins, present in hostile extracellular conditions, need long half‐lives in order to accumulate and, hence, be detected. Protein translocation through the cytoplasmic membrane is believed to be mainly driven by the Sec and Tat pathways (see e.g. Barnett et al., 2011 for a review of the cyanobacterial Tat system), while up to eight different translocation systems carry proteins through the cytoplasmic and outer membranes of gram‐negative bacteria (Saier, 2006), three of which secrete proteins directly though the gram‐negative bilayer (type I, III and IV). To date, extensive exoproteomes of environmental bacteria are poorly described, particularly for marine microorganisms, with only two single case studies in the γ‐proteobacterium Pseudoalteromonas tunicata and the α‐proteobacterium Ruegeria pomeroyi (Evans et al., 2007; Christie‐Oleza and Armengaud, 2010), and a comprehensive analysis of marine Roseobacters (Christie‐Oleza et al., 2012). This latter analysis of the exoproteome among members of the Roseobacter clade showed different adaptive life strategies among the studied strains inferred by their exported proteins (Christie‐Oleza et al., 2012).
Picocyanobacteria comprise a key component of the marine picoplankton being major contributors to primary production and underpinning the marine food web due to their large abundance (Partensky et al., 1999; Jardillier et al., 2010). Two genera, Prochlorococcus and Synechococcus, numerically dominate marine waters, occupying complementary although overlapping oceanic regimes and with both genera exhibiting extensive genomic diversity (see Partensky et al., 1999; Kettler et al., 2007; Scanlan et al., 2009; Kashtan et al., 2014). The existence of hypervariable genomic regions (or islands) (Palenik et al., 2003; Coleman et al., 2006; Dufresne et al., 2008) has been linked with the importance of these regions in environmental adaptation (both biotic and abiotic) in both genera (Avrani et al., 2011; Stuart et al., 2013). Furthermore, closely related coexisting Prochlorococcus subpopulations according to their intergenic transcribed spacer showed a much higher divergence in terms of their genomic‐encoded auxilliary functions as a matter of avoiding competition and niche partitioning (Kashtan et al., 2014). Hence, the existing diversity of functions encoded by this group of photosynthetic organisms is somewhat far from being understood.
While numerous transcriptomic experiments have been performed on marine picocyanobacteria, studies using high‐throughput proteomics are more limited, e.g. quantitative proteomics of Prochlorococcus have been performed during light adaptation (Pandhal et al., 2007), a light‐dark cycle (Waldbauer et al., 2012), nitrogen starvation conditions (McDonagh et al., 2012) or from extracellular vesicles (Biller et al., 2014), while temperature shifts (Mackey et al., 2013) and nutrient depletion proteomes (Cox and Saito, 2013) are available for Synechococcus. However, very little is known with respect to exported proteins of marine picocyanobacteria with a few exceptions, e.g. the swimming and grazing defence proteins SwmA and SwmB in Synechococcus sp. WH8102 (McCarren and Brahamsha, 2007; Strom et al., 2012) and the PhoX alkaline phosphatase (Kathuria and Martiny, 2011). Here, we present the first exported pan‐proteome analysis of marine picocyanobacteria. Our initial bioinformatic prediction of the theoretical exoproteomes for a number of Synechococcus and Prochlorococcus strains clearly highlights the exported fraction as the major reservoir of proteins of unknown function and shows largest variability between strains. These observations suggest that the major discriminating parameter between strains lies within their exoproteome. Subsequent experimental analysis of the exoproteomes of eight Synechococcus strains revealed not only the expected importance of nutrient transport systems (e.g. those for phosphorus and iron) to these organisms, but also demonstrated expression of a wide range of poorly conserved exoenzymes with community interacting and mixotrophic implications. Both the need for novelty in the adaptation and survival of each strain in its specific environment and the weaker evolutionary constraints on proteins outside of cells may explain this pan‐exoproteome diversity.
Results
Pan‐genome analysis of the theoretical exoproteome of marine picocyanobacteria
The exported proteins encoded by eight Synechococcus strains, encompassing members of several clades, four Prochlorococcus strains (encompassing two high light‐ and two low light‐adapted) and Candidatus Pelagibacter ubique (SAR11 in the text) were predicted using the prediction tools SignalP, SecretomeP, LipoP and PSORTb (Table S1). Table 1 reports the number of predicted exported proteins and their ratio compared with the whole theoretical proteome for each strain. These analyses showed that almost 40% of the total products of coding DNA sequences (CDS) of these organisms were predicted to be exported to the membrane or extracellular milieu. Figure 1A shows the ratio of uncharacterized proteins for the 12 picocyanobacteria. Remarkably, the proportion of proteins annotated as ‘hypothetical protein’ or with unknown function within the theoretically exported fraction (54 ± 7%) exceeded the overall theoretical proteome average (43 ± 7%) and was much higher than within the cytoplasmic fraction (35 ± 7%) (Fig. 1A).
Table 1.
Theoretical exoproteomes
| Total CDS | Cytoplasmic fraction | Exported fraction | ||||
|---|---|---|---|---|---|---|
| CDS | % | CDS | % | |||
| Synechococcus | BL107 | 2507 | 1563 | 62 | 941 | 38 |
| CC9311 | 2892 | 1676 | 58 | 1216 | 42 | |
| RS9916 | 2961 | 1620 | 55 | 1341 | 45 | |
| RS9917 | 2770 | 1717 | 62 | 1053 | 38 | |
| WH5701 | 3346 | 2044 | 61 | 1302 | 39 | |
| WH7803 | 2533 | 1482 | 59 | 1051 | 41 | |
| WH7805 | 2883 | 1702 | 59 | 1181 | 41 | |
| WH8102 | 2519 | 1604 | 64 | 915 | 36 | |
| Prochlorococcus | MED4 | 1717 | 1104 | 64 | 613 | 36 |
| MIT9303 | 2997 | 1676 | 56 | 1321 | 44 | |
| MIT9312 | 1810 | 1160 | 64 | 650 | 36 | |
| MIT9313 | 2269 | 1352 | 60 | 917 | 40 | |
| Ca. P. ubique | SAR11 | 1354 | 800 | 59 | 554 | 41 |
| Average | 60 ± 3 | 40 ± 3 | ||||
Figure 1.
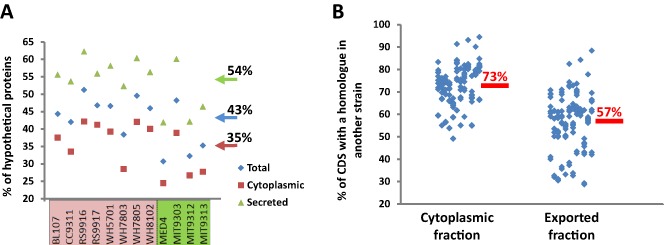
Theoretical exoproteomes of twelve marine picocyanobacterial strains. (A) Percentage of CDS annotated as hypothetical proteins in the cellular fractions (cytoplasmic and exported) of eight S ynechococcus strains (pink) and four P rochlorococcus strains (green). (B) Percentage of CDS from each strain and fraction (cytoplasmic and exported fractions) that found a homologous protein in each of the other eleven strains analysed in this study. Data were extracted from Table S2.
A homology table where the CDS of each marine picocyanobacterium was searched against all other 11 strains used in this study (considering only BLASTp hits with an E‐value < 10−20) was constructed (Table S2). Figure 1B depicts for both fractions the global similarity level of their components. A considerably lower number of predicted exported CDS found a homologue in another strain (57% on average) when compared with the cytoplasmic fraction (73% on average), which highlights the ‘distinctness’ the exported protein fraction confers to individual strains (Fig. 1B). Even closely related Synechococcus strains such as WH7803 and WH7805 or high‐light Prochlorococcus MIT9312 and MED4 showed a higher proportion of homologous hits in the cytoplasmic fraction (91% and 95% respectively) as opposed to the exported protein fraction (82% and 88%).
Table 2 shows the functional grouping of proteins in eight clusters for the 12 picocyanobacteria. Proteins included in each cluster can be found in Table S2 and are further described in the Appendix S1.
Table 2.
Functional grouping of picocyanobacterial theoretical exoproteomes
| Synechococcus | Prochlorococcus | % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL107 | CC9311 | RS9916 | RS9917 | WH5701 | WH7803 | WH7805 | WH8102 | MED4 | MIT9303 | MIT9312 | MIT9313 | ||
| Transport systems | 99 | 139 | 121 | 152 | 165 | 152 | 143 | 120 | 67 | 120 | 75 | 116 | 11.8 |
| Cell wall structure/biogenesis | 39 | 42 | 40 | 35 | 49 | 45 | 41 | 40 | 28 | 39 | 28 | 42 | 3.9 |
| Photosynthesis/energy production | 101 | 109 | 100 | 95 | 100 | 101 | 99 | 100 | 82 | 86 | 81 | 88 | 9.6 |
| Coenzyme metabolism | 20 | 17 | 18 | 17 | 23 | 16 | 19 | 16 | 17 | 17 | 14 | 18 | 1.8 |
| Oxidative stress | 15 | 16 | 15 | 14 | 18 | 16 | 16 | 13 | 12 | 14 | 11 | 15 | 1.4 |
| Interaction and environment sensing | 18 | 31 | 38 | 29 | 20 | 33 | 25 | 22 | 6 | 27 | 9 | 24 | 2.1 |
| Others | 140 | 230 | 211 | 171 | 206 | 159 | 162 | 115 | 160 | 259 | 167 | 217 | 18.3 |
| Hypothetical/unknown | 509 | 632 | 798 | 540 | 721 | 529 | 676 | 489 | 241 | 759 | 265 | 397 | 51.2 |
| Total exported | 941 | 1216 | 1341 | 1053 | 1302 | 1051 | 1181 | 915 | 613 | 1321 | 650 | 917 | – |
Comparative experimental exoproteomes of eight S ynechococcus strains
Figure 2 shows the SDS‐PAGE‐resolved exoproteomes of the eight Synechococcus strains. We observed that Synechococcus cultures generally accumulated proteins in the milieu in stationary phase possibly as a consequence of debris accumulation, especially in axenic cultures (Fig. 2A). Hence, cells were washed prior to transfer to avoid the carryover of proteins. Exoproteomes were subsequently prepared from culture supernatants (see Experimental procedures) following an increase in cell numbers from 3 × 107 to 1 × 108 cells ml−1 (see Fig. 2B where the equivalent of 40 ml of concentrated exoproteome from each strain was analysed by SDS‐PAGE). Shotgun nanoLC‐MS/MS proteomic analysis resulted in a total of 25 920 tryptic peptides being detected. Table 3 shows the number of non‐redundant peptides and proteins detected for each strain, as well as their functional categorization. We evaluated the fraction of proteins in the exoproteome that potentially originated from cell lysis by following the presence of ribosomal proteins. In all non‐axenic strains these proteins were not detected, while only a very low fraction was detected in the axenic strains (e.g. 1.1% in Synechococcus sp. WH8102; Table 3). For comparison, analysis of intracellular proteomes obtained from Synechococcus sp. WH8102 using the same culture conditions showed a much greater abundance of ribosomal proteins, comprising 8.8% of the proteome (data not shown) suggesting that even in the worst‐case scenario, < 13% of the exoproteomes could result from cell lysis under the conditions tested here. The non‐axenic Synechococcus strains produced a lower number of detected polypeptides as was evident from the SDS‐PAGE analysis (Fig. 2). Synechococcus sp. BL107 was the strain with the smallest number of polypeptides detected (11) despite re‐running samples through the mass spectrometer with four times more material. Synechococcus sp. BL107, unlike all other strains, showed almost no accumulation of proteins in the milieu (Fig. 2A). In contrast, a considerably higher number of polypeptides were detected in the axenic strains (WH5701, WH7803, WH7805 and WH8102), e.g. 247 polypeptides in Synechococcus sp. WH5701. Interestingly, 82% of the non‐axenic exoproteomes were predicted exported polypeptides whereas this was only 53% in the case of axenic strains. It is possible that the turnover of cytoplasmic proteins is higher in the extracellular milieu because these proteins may be potential targets for the exoprotease activity of heterotrophic bacteria as we discuss below. The detailed results of comparative exoproteomics between the eight strains are reported in Table S4 and commented on hereafter.
Figure 2.
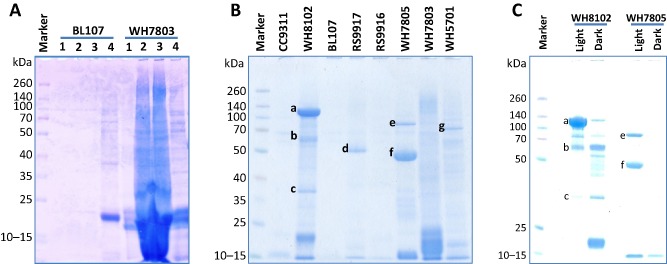
Exoproteomes of different marine S ynechococcus strains. Concentrated exoproteomes equivalent to 40 ml of culture were resolved by 10% Tris‐Bis NuPAGE gel (Invitrogen) and stained with coomassie G‐250 (SimplyBlue SafeStain, Invitrogen). Spectra Multicolor Broad Range Protein Ladder (Fermentas) was used as a marker. (A) Process for optimizing exoproteome preparation. The exoproteomes from S ynechococcus spp. BL107 and WH7803 were prepared from cultures: lane 1, washed cells in fresh ASW (107 cells ml−1) and incubated for 2 days; lane 2, routinely subbed cultures (15% volume from an older culture in fresh ASW medium, resulting in 107 cells ml−1) incubated for 2 days; lane 3, washed cells in fresh ASW (107 cells ml−1) and incubated for 14 days; lane 4, protein extract of the cellular fraction from the corresponding S ynechococcus strain. (B) Exoproteomes from each one of the eight S ynechococcus strains analysed in this study by shotgun proteomics. Preparation of the exoproteomes was as indicated in the section E xperimental procedures. Letters indicate those resolved protein bands that were further identified using chymotrypsin digestion prior to LC‐MS‐MS: (a) SwmA NP_896180.1; (b) SwmA NP_896180.1 and SwmB NP_897046.1; (c) substrate binding protein of a phosphate ABC transporter NP_897111.1; (d) chitinase ZP_01081204.1; (e) type I secretion protein EAR18050.1; (f) haemolysin EAR19380.1 and chitinase EAR19694.1; (g) alkaline phosphatase EAQ75607.1. (C) Exoproteomes from light/dark incubated cultures of S ynechococcus sp. WH8102 and WH7805. Bands a, b, c, e and f are as those highlighted in panel B.
Table 3.
Proteins detected by shotgun proteomics in the exoproteomes of eight marine S ynechococcus strains and abundance of these proteins across different functional groups
| Non‐axenic cultures | Axenic cultures | |||||||
|---|---|---|---|---|---|---|---|---|
| BL107 | CC9311 | RS9916 | RS9917 | WH5701 | WH7803 | WH7805 | WH8102 | |
| Peptides (LC‐MS/MS runs) | 340 (4) | 1180 (2) | 851 (2) | 2325 (2) | 7422 (2) | 5420 (2) | 2352 (2) | 6030 (3) |
| Non‐redundant peptides | 74 | 371 | 295 | 531 | 2071 | 1414 | 656 | 1231 |
| Validated proteins (≥ 2 peptides) | 11 | 60 | 42 | 67 | 247 | 202 | 84 | 146 |
| Non‐exported | 3.2% | 38.7% | 15.8% | 14.1% | 57.5% | 44.6% | 34.0% | 52.2% |
| Ribosomal proteins | 0.0% | 0.0% | 0.0% | 0.0% | 1.2% | 0.6% | 0.0% | 1.1% |
| Exported | 96.8% | 61.3% | 84.2% | 85.9% | 42.5% | 55.4% | 66.0% | 47.8% |
| Transport systems | 10.4% | 5.0% | 13.9% | 9.2% | 5.2% | 0.5% | 10.0% | 6.5% |
| Cell wall structure/biogenesis | 0.0% | 0.4% | 0.0% | 2.2% | 0.8% | 0.5% | 0.2% | 0.5% |
| Photosynthesis/energy production | 56.8% | 17.8% | 36.0% | 7.8% | 11.2% | 18.0% | 5.7% | 19.9% |
| Oxidative stress | 0.0% | 1.3% | 3.0% | 4.1% | 3.4% | 3.9% | 6.7% | 5.2% |
| Interaction and environment sensing | 16.5% | 8.7% | 7.7% | 11.0% | 2.3% | 20.1% | 8.6% | 3.0% |
| Others | 0.0% | 14.2% | 2.7% | 11.8% | 7.6% | 4.2% | 8.6% | 4.7% |
| Hypothetical/unknown | 13.1% | 14.0% | 20.9% | 39.8% | 12.1% | 8.1% | 26.0% | 8.1% |
Photosynthesis/energy production
Despite being proteins targeting the thylakoid membrane, this functional group of proteins is the most abundantly detected among the exoproteomes of Synechococcus (Table 3). This is principally due to the accumulation of their light harvesting pigments and associated phycobilisomes in the milieu (Table S4). For example, the four C‐phycoerythrin polypeptides (alpha/beta chains of class I/II C‐phycoerythrin, between 18–24 kDa in size; WH7805 only encodes class I) represent on average 26% of the exoproteome in the six strains in which they are encoded. Given the low abundance of ribosomal proteins (i.e. cytoplasmic non‐exported proteins) in our exoproteomes, suggesting low cell lysis, it is difficult to explain the presence of phycobilisome components in this fraction. The protein composition and dynamics in the plasma and thylakoid membrane systems remains a major challenge in cyanobacterial cell biology (Schneider, 2014) and, hence, it is possible these proteins are a result of mis‐targeting to the plasma membrane and beyond or if this is a consequence of thylakoid membrane blebbing and/or the production of extracellular vesicles (Biller et al., 2014).
Transport systems
Proteins involved in transporting substrates across the cell membrane were commonly found in the exoproteomes of Synechococcus, mainly ABC transporters and porins. ABC transporters specialized in transporting essential inorganic nutrients such as iron, phosphorus and, to a lesser extent, nitrogen were most abundant. The substrate binding protein of the iron ABC transporter was, on average, the sixth most abundant detected protein in our study mainly as it represented 8.7% and 4.6% of the exoproteome of strains RS9916 and RS9917 respectively (Table S4). The substrate binding protein of the phosphate ABC transporter was the seventh most abundant protein (Table S4) despite it being only abundantly detected in the axenic strains and the only transporter‐like protein detected in Synechococcus sp. WH7803. Interestingly, different isoforms of the phosphate binding protein were detected in strains WH5701, WH7805 and WH8102, suggesting that they may have subtly different functional roles, e.g. different binding affinities for phosphate. A potential component of a phosphonate ABC transport protein was detected (Table S4) in strains RS9917, WH5701 and WH7805 (the corresponding gene is conserved across picocyanobacterial genomes, see Scanlan et al., 2009) despite the fact that C‐P lyase, the enzyme required to cleave the recalcitrant C‐P bond of these compounds, has not been identified in any Synechococcus genome. Transporters for nitrogen were less abundant possibly because of the high N : P ratio (50:1) in ASW medium repressing their expression. Curiously, a potential urea ABC transporter protein was the most abundantly detected nitrogen transporter despite the fact that nitrate was the sole N source in ASW medium.
Oxidative stress
Proteins involved in oxidative stress were detected in the different exoproteomic fractions (Table 3). Superoxide dismutase (SOD), involved in detoxifying oxygen radicals, was detected in all strains except for BL107. Interestingly, all strains encoding the iron‐containing SOD produced this isoform (cluster 15 in Table S4). Those strains that lack the iron isoform of SOD, CC9311 and WH8102 produced the Cu/Zn and Ni isoforms respectively (cluster 106 and 99 in Table S4). Between one and three different thioredoxin peroxidase isoforms, with a function in cleaving hydrogen peroxide, were detected in all strains except for strains BL107 and CC9311 (clusters 21, 31, 232 and 287, Table S4). In Synechococcus sp. WH8102 however, we also detected a rubrerythrin‐like protein (comprising 2.9% of the exoproteome of this strain) playing a potential role in reducing hydrogen peroxide (Sztukowska et al., 2002).
Interaction and environment sensing
This group of proteins shows high strain‐specificity as detected proteins are generally unique to a particular strain or with only a few homologues. However, two of these exoproteins that do show a high degree of conservation are: (i) an abundant metallo‐β‐lactamase (with 1.3% average detection excluding BL107, cluster 16 in Table S4) with high identity across currently sequenced picocyanobacterial genomes (Fig. 3A) and (ii) the third most abundant polypeptide detected in our exoproteome survey (cluster 3, Table S4), annotated as a potential protein phosphatase 2C, that may play a key role in signal transduction (Fuchs et al., 2013). Interestingly, this protein phosphatase 2C is highly conserved and encoded by all eight Synechococcus strains.
Figure 3.
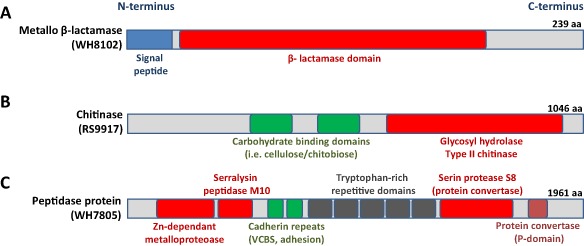
Domain structure of relevant exoproteins detected in marine S ynechococcus strains. (A) Highly conserved metallo β‐lactamase‐like protein abundantly detected in the exoproteomes of seven of the eight S ynechococcus strains analysed (NP_898405.1). (B) Abundantly detected chitinase‐like exoenzyme found in S ynechococcus spp. WH7803, WH7805, RS9916, RS9917, all with a similar domain structure (ZP_01081204.1). (C) Large protease‐like protein abundantly detected and unique to Synechococcus sp. WH7805 (EAR17767.1).
Surprisingly, we abundantly detected a potential endo‐1–4‐β‐glycosyl hydrolase (a type II chitinase, cluster 12 in Table S4) in all those Synechococcus strains that encode this exoenzyme (i.e. 8.5% in RS9917; 2.0% in WH7805; 1.4% in RS9916; and 0.1% in WH7803) despite no substrate (chitin or similar) being added to the growth medium. All encoded chitinases display a similar structure, with carbohydrate‐binding domains at the N‐terminal half of the protein and a type II chitinase domain at the C‐terminus (Fig. 3B). These exoproteins have no signal peptide and are predicted to use a non‐classical mechanism for secretion. Interestingly, the gene encoding the Synechococcus sp. WH7803 chitinase lies immediately upstream of another annotated chitinase that was detected in similar abundance in the exoproteome of this strain (YP_001225792.1). Complete degradation of chitin requires β‐N‐acetylglucosaminidase (Gooday, 1990), although this enzyme was not detected in the experimental exoproteomes. β‐N‐acetylglucosaminidase is encoded in all of the picocyanobacterial genomes analysed in this study (except for the two HL Prochlorococcus), but is predicted to be a non‐exported protein, in accordance with its absence in the proteomics data.
The exoproteome of Synechococcus sp. WH7803 contained an extremely abundant pili‐like structure composed of proteins YP_001225518.1 and YP_001225519.1 representing 17.9% and 1.3% of the exoproteome respectively. No pili‐like proteins were detected in other strains. The motility proteins SwmA and SwmB from Synechococcus sp. WH8102 (McCarren and Brahamsha, 2007) comprised 1.7% and 0.13%, respectively, of the exoproteome of this strain. The giant protein SwmB (10 791 amino acids in length) was previously visualized in the membrane fraction (McCarren and Brahamsha, 2007) but not resolved in Fig. 2B. This protein shared a low identity with the MS‐detected protein EAU73526 in Synechococcus sp. RS9916 (0.15% of the exoproteome), a much smaller protein of only 1159 amino acids in length but which contains a conserved flagellar‐like domain. Seven other giant proteins (> 2000 amino acids in length) were also detected during this study and are further discussed in the Appendix S1. The large protein, EAR17767.1 (1961 amino acids in length; 0.67% of the exoproteome), from Synechococcus sp. WH7805 contains up to three different peptidase domains, two cadherin adhesion repeats and five tryptophan‐rich repetitive domains, highlighting the complexity of these large proteins (Fig. 3C). Protein EAR19380.1, abundantly detected (2.2%) and unique to Synechococcus sp. WH7805, also contained a serralysin peptidase M10 domain, which commonly binds calcium in haemolysin‐like proteins (pfam08548).
Other functions
Alkaline phosphatase enzymes were only detected in Synechococcus sp. WH5701 and Synechococcus sp. WH8102. While Synechococcus sp. WH5701 contained only one alkaline phosphatase in its exoproteome (EAQ75607.1, third most detected protein, 1.9%), Synechococcus sp. WH8102 expressed three different potential isoenzymes (NP_898480.1, 1.8%; NP_898479.1, 0.4%; and NP_896291.1, 0.1%) and a phytase‐like protein (NP_896855.1, 0.3%).
Exported hypothetical proteins
This group of unknown proteins, representing on average 18% of the experimental exoproteomes across all eight strains analysed, but rising to almost 40% of the exoproteome of Synechococcus sp. RS9917 (Table 3), comprises proteins of a relatively small size (150–240 amino acids in length). Interestingly, we found abundantly exported hypothetical proteins that are present in the genomes of most marine picocyanobacteria (e.g. protein cluster 8 and 18 of Table S4) or that were specific to the genus Synechococcus (e.g. clusters 17 and 24 of Table S4), all being abundantly detected in most of the analysed exoproteomes. Other highly detected exported hypothetical proteins showed a higher strain‐specificity, e.g. clusters 19 (8.9% abundance) and 35 (4.7%) were specific to strains BL107 and RS9917 respectively.
We further verified the identity of the most abundant proteins in Synechococcus exoproteomes seen in Fig. 2B using chymotrypsin digestion. This highlighted the importance of the swimming proteins SwmA and SwmB in Synechococcus sp. WH8102, the chitinase‐like enzyme in Synechococcus sp. RS9917 and WH7805, and the haemolysin‐related proteins in Synechococcus sp. WH7805 (further details on this analysis can be found in the Appendix S1).
Characterization of the S ynechococcus WH8102 exoproteome following growth under different environmental conditions
In addition to growth of (i) Synechococcus WH8102 over a 4 day period under the standard conditions mentioned above, exoproteomes were also analysed following (ii) co‐culture of WH8102 with the α‐proteobacterium R. pomeroyi DSS‐3, (iii) growth of WH8102 over a 10 h incubation period, (iv) 10 h dark incubation of WH8102 and (v) WH8102 cultures infected for 10 h with cyanophage S‐RSM4. Shotgun proteomic analysis of these five different conditions (each performed in triplicate) generated 24 914 MS/MS‐identified peptides. In this case, on average, 101 proteins per sample were validated (Table S5). Comparative proteomics between each of the relevant conditions was carried out (Table S6) and are commented upon hereafter.
Stand‐alone growth versus co‐culture growth
Co‐culture with R. pomeroyi revealed strong upregulation of four hypothetical proteins under co‐culture conditions: NP_896260.1 with no known function (8.4×); and NP_898392.1 (5.2×), NP_896974.1 (5.1×) and NP_898441.1 (4.3×), which contained domains related to virulent factor proteases and adhesion. These latter three proteins together with two haemolysin‐like proteins that were also upregulated in the presence of R. pomeroyi DSS‐3 (NP_898382.1, 3.3×; NP_898498.1, 1.8×) are indicative of a predator‐type response by Synechococcus sp. WH8102 to the presence of the heterotroph. The haemolysin‐like protein (NP_898382.1) also contains an alpha‐tubulin suppressor‐like domain, as do two other proteins exported under co‐culture conditions (NP_897657.1, 2.6×; NP_897658.1, 2.4×), which potentially hints at a role in restructuring the cell surface envelope in Synechococcus sp. WH8102. The motility proteins SwmA and SwmB were also upregulated (NP_896180.1, 1.7×; NP_897046.1, 3.0×) together with three alkaline phosphatases (NP_896291.1, 4.1×; NP_898479.1, 2.2×; NP_898480.1, 2.1×) and a phytase‐like protein (NP_896855.1, 3.2×), the latter proteins, at least, suggesting phosphate depletion as was seen previously when this strain was grown in the presence of Vibrio parahaemolyticus (Tai et al., 2009). Unlike the upregulated proteins, which were all potentially exported proteins, downregulated proteins found in the exoproteome were mostly cytoplasmic, i.e. lacked a specific signal sequence (43 out of 56, Table S6).
Standard (4 days) versus shorter incubation time (10 h)
As expected, a reduced number and lower concentration of leaked proteins (i.e. downregulation of numerous cytoplasmic proteins) occurred with shorter incubation time. Interestingly, two porins were strongly upregulated (NP_898316.1, 15.4×; NP_898315.1, 6.9×) together with active ABC transporters for iron and urea (9.8× and 2.9× respectively).
Standard versus dark conditions
Upregulated exoproteins under dark conditions were mostly predicted cytoplasmic proteins indicating an increased leakage of proteins to the milieu (36 out of 46, Table S6). The three abundantly detected alkaline phosphatases, a phytase‐like enzyme and the motility proteins SwmA and SwmB were strongly downregulated and almost undetectable under dark conditions. Noteworthy was the downregulation of all the interaction proteins abundantly observed when co‐cultured with the heterotroph (i.e. the three proteins containing alpha‐tubulin suppressor domains, haemolysins and metallopeptidases). This was visually confirmed by SDS‐PAGE and noted in other strains, i.e. Synechococcus sp. WH7805 where bands e and f of Fig. 3C containing type I secretion protein (EAR18050.1), haemolysin (EAR19380.1) and chitinase (EAR19694.1) disappeared.
Standard versus phage infection
A low number of exoproteins showed a variation in abundance following infection with cyanophage S‐RSM4. The motility proteins SwmA and SwmB showed a moderate downregulation (2.2× and 2.0× respectively), while the already abundantly detected substrate binding proteins of the phosphate ABC transporter NP_897906.1 and NP_897111.1 showed an increase (2.0× and 1.3× respectively).
S ynechococcus co‐cultures with heterotrophs
All of our data indicated a progressive accumulation of cytoplasmic proteins in the exoproteomes of axenic cultures but not in non‐axenic Synechococcus cultures, i.e. the non‐exported proteins detected in the exoproteomes of Synechococcus sp. WH8102 increased from 3.3 ± 0.6% after 10 h incubations to 52.2 ± 0.8% after 4 day incubations, but for the latter this was reduced to 33.2 ± 2.4% when R. pomeroyi DSS‐3 was present in the culture. We monitored the accumulation of proteins in the exoproteomes of all eight analysed Synechococcus strains (Fig. 4A). As expected, elevated concentrations of proteins in the exoproteome were only observed in axenic Synechococcus cultures during the stationary phase of growth (between 19 and 42 μg ml−1 depending on strain). Interestingly, the concentration of exoproteins in axenic Synechococcus sp. WH7803 supernatants was reduced threefold when grown with R. pomeroyi DSS‐3 (27.9 versus 9.2 μg ml−1), the latter concentration comparable with that seen in other non‐axenic Synechococcus cultures (Fig. 4A). Growth of Synechococcus sp. WH7803 in axenic versus R. pomeroyi DSS‐3 co‐culture (Fig. 4B) was very similar when monitored by cell counting, with doubling times of 38.2 and 40.1 h, respectively, and comparable cell yields after 28 days of incubation (6.4 × 108 and 6.2 × 108 cells l−1 respectively). These results indicate that the higher number of predicted cytoplasmic proteins in the exoproteomes and the higher concentration of protein accumulated in stationary phase is not due to an increase in cell lysis of axenic cultures, but to a faster turnover of these proteins when heterotrophic bacteria are present.
Figure 4.
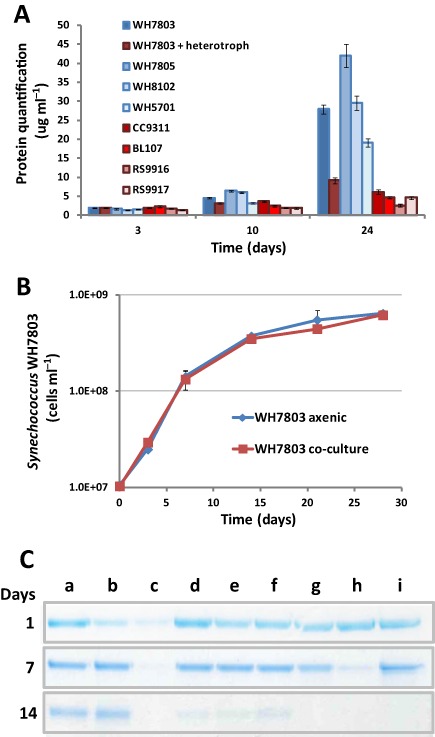
S ynechococcus‐heterotroph co‐culturing. (A) Quantification of proteins accumulated in the milieu of the eight different S ynechoccus strains used in this study. Bars are an average of three biological replicates. Red tones represent cultures where heterotrophs were present and blue tones represent axenic cultures. (B) Growth of axenic S ynechococcus sp. WH7803 under standard conditions and in co‐culture with R . pomeroyi DSS‐3. (C) BSA degradation in different culture conditions: (a) ASW control; (b) culture with S ynechococcus sp. WH7803; (c) co‐culture with S ynechococcus sp. WH7803 and R . pomeroyi DSS‐3; (d–i) R. pomeroyi DSS‐3 cultured in media: (d) ASW; (e) ASW supplemented with NH4 (9 mM); (f) ASW with yeast extract (0.005%); (g) ASW with yeast extract and pyruvate (0.5%); (h) 1/10 marine broth in ASW; (i) marine broth. BSA was used at a final concentration of 0.02% w/v). Cultures were filtered through a 0.22 μm pore size membrane and proteins from 20 μl of filtered milieu were resolved by 10% SDS‐PAGE.
Finally, we analysed the proteolytic activity of Synechococcus sp. WH7803 and R. pomeroyi DSS‐3 by monitoring the degradation of BSA added to the culture. The results reported in Fig. 4C show that Synechococcus sp. WH7803 was unable to hydrolyse BSA after 14 days incubation, whereas R. pomeroyi DSS‐3 showed almost complete degradation of the protein. Most interestingly, the fastest degradation of BSA was seen in R. pomeroyi DSS‐3‐Synechococcus sp. WH7803 co‐cultures (Fig. 4C, lane c).
Discussion
Exoproteomes are good indicators of microbial lifestyle strategies. Here, we carried out a thorough theoretical and experimental analysis of marine picocyanobacterial exoproteomes. The predicted exported fraction of these key photosynthetic primary producers (40% of their encoded proteins) highlighted two major points: (i) the current lack of functional knowledge regarding exported proteins (54% of the theoretical exported CDS are annotated as ‘hypothetical proteins’ whereas these unknown proteins only represented 35% of the cytosolic fraction) and (ii) this exported fraction appears to be a major factor in dictating the genomic and functional distinctness of these closely related organisms (only 57% of the exported CDS contain a homologue in other Synechococcus stains versus 73% in the cytosolic fraction). Exposure to the outside world determines their strategy for interaction, and ultimately accomplishes niche partitioning of closely related organisms not only to avoid direct competition, but also to evade grazing or phage infection. For example, diversification of potentially membrane‐exposed proteins, which might act as phage receptors, across closely related strains would avoid catastrophic lysing of members of the entire genus at any given time and location. In this study, we used model strains isolated at different times and locations, but recently published data showing the distinctness in coexisting Prochlorococcus subpopulations (Kashtan et al., 2014) shows how this may actually be relevant in natural environments.
Experimental analysis of eight marine Synechococcus strains confirmed how each strain has a distinct adaptation through the exported protein fraction. For example, we were surprised that the pili‐like structure encoded by four of the strains was only detected in Synechococcus sp. WH7803 and represented as much as 19% of this strains exoproteome. Exported proteins involved in microbial interactions showed the highest diversification, with many of the genes encoding these proteins unique to specific strains. Other previously published examples include small ribosomally synthesized peptides with toxic effects (Paz‐Yepes et al., 2013; Zhang et al., 2014) through to giant proteins involved in motility and grazing evasion (McCarren and Brahamsha, 2007; Strom et al., 2012). Giant proteins were abundantly detected in our MS/MS analysis although became less relevant after normalizing the data to their molecular size. These giant proteins (up to four different ones detected in Synechococcus sp. RS9916) were classified as interaction proteins because of the adhesion and haemolysin‐like domains they contain. This diverse set of peculiar large proteins, that rarely share similarities among strains, are thought to play a role in shielding cells from potential threats (Scanlan et al., 2009).
Evolution of intracellular proteins is restrained because of protein crowding and numerous protein–protein interactions in the cell that may well not occur in exported proteins, and consequently their evolution is more prone to be highly divergent. Furthermore, extracellular enzymes with a simple genetic structure are known to be rapidly gained and lost through evolution (Zimmerman et al., 2013), facilitating the acquisition of functional distinctness via this extracellular fraction. Horizontal gene transfer of large genetic islands may facilitate the acquisition of these simple functions, but also of more complex operons or genes encoding for giant proteins such as those detected here (Dufresne et al., 2008). In this respect, the abundant detection of a chitinase‐like endo‐1,4‐beta‐glucanase (in the exoproteomes of Synechococcus spp. RS9916, RS9917, WH7803 and WH7805) and potential exoproteases (e.g. EAR17767.1 produced by Synechococcus sp. WH7805) may have been acquired and conserved as a result of a beneficial selection. It is interesting that microorganisms such as marine Synechococcus, with a known preference for inorganic nutrients, produce exo‐chitinases and proteases that, ultimately, may have a role in nutrient supplementation (i.e. as additional nitrogen sources) as part of a mixotrophic lifestyle. Indeed, picocyanobacteria are known for their ability to acquire amino acids and carbohydrates (Montesinos et al., 1997; Zubkov et al., 2003; Mary et al., 2008; Muñoz‐Marín et al., 2013) at low nanomolar concentrations, potential products of chitinase or protease activity. It is also possible that these exoenzymes may have a role in eliminating competitors, especially diatoms, because the latter contain chitin in their silica cell wall (Brunner et al., 2009). Type IV pilins, like the one detected in Synechococcus sp. WH7803, have also been reported to have a potential role in chitin adhesion in Vibrio (Frischkorn et al., 2013). Hence, picocyanobacteria may appear to be more hostile microorganisms than previously anticipated.
The acquisition of inorganic nutrients is a key process carried out at the cellular membrane of marine cyanobacteria. Proteins involved in transport are commonly detected in exoproteome studies (Christie‐Oleza and Armengaud, 2010; Johnson‐Rollings et al., 2014), and, therefore, it was not surprising to find that substrate binding proteins of ABC transporters for iron and phosphate were among the most abundant proteins detected over the entire study. The fact that different isoforms of the phosphate binding protein component of the ABC transporter and several alkaline phosphatases co‐occurred in some of the Synechococcus exoproteomes suggests the experimental cultures were actively scavenging P from their environment, likely both for growth and storage (Moore et al., 2005; Mazard et al., 2012).
Overall, our experimental data indicate that non‐exported proteins of Synechococcus can be found in the extracellular milieu. Whether the observed leakage is via cell lysis, unknown cell loss mechanisms or by the large quantity of vesicles these organisms are known to release (Biller et al., 2014) is unclear. Whatever the mechanism, axenic cultures accumulate larger amounts of cytoplasmic and thylakoid‐like proteins in their milieu than non‐axenic cultures. This could be due to a lower incidence of Synechococcus cell lysis when co‐cultured with a heterotroph, or that the heterotrophic bacteria present in co‐cultures are capable of degrading the more labile cytoplasmic proteins and, hence, these have a faster turnover in the milieu. Despite the difficulty to experimentally ascertain which is the most likely scenario, the identical growth rate seen in axenic and co‐cultured Synechococcus indicates similar cell lysis rates. Interestingly, the heterotroph R. pomeroyi DSS‐3 showed high exoprotease activity when grown in co‐culture with Synechococcus, indicating increased potential for removing proteins from the milieu. Picocyanobacterial cell debris and vesicles are known to support heterotrophic growth (Biller et al., 2014), perhaps via some type of mutualistic interaction. The presence of a heterotroph not only removed leaked cytoplasmic proteins from the exoproteome, but in Synechococcus sp. WH8102 also caused the upregulation of a large set of interaction proteins (i.e. swimming proteins, virulent factor‐like proteases, haemolysin and adhesion proteins). The other condition tested which showed a strong influence on the exoproteome was dark incubations. Probably pushed by a decrease in its energy potential, Synechococcus drastically reduced protein export, especially those with roles in interaction (i.e. involved in swimming, haemolysin production, alkaline phosphatase, see Fig. 2C). This form of dormancy could be a strategy not only to save energy, but may also disguise cells at night by reducing the prevalence of viral receptors and grazer recognition proteins, being a possible explanation as to why some cyanophage are unable to absorb their host in the dark.
Our experimental exoproteomes draw some parallels to those observed in the natural environment from a metaproteome of high molecular weight dissolved organic matter in surface seawaters of the South China Sea (Dong et al., 2013). Despite the fact that only 17 of 367 identified polypeptides could be confidently assigned to cyanobacteria, eight were of unknown function, two were nitrogen membrane transporters, and four were directly involved in photosynthesis and carbon fixation.
In conclusion, the exoproteomes of Synechococcus highlight several particularly interesting ecological traits of marine picocyanobacteria: (i) the exported fraction gives a specific functional distinctness to these phototrophs for potential niche partitioning and diversifying receptors to evade prey, (ii) the large number of uncharacterized proteins these organisms have to interact with their environment, (iii) the potential hostile repertoire of exoenzymes for eliminating direct competitors or to supplement nutritional needs through mixotrophy, (iv) their ability to strongly modify their exoproteome under different conditions, i.e. light/dark or growth with heterotrophs, and (v) how the exoproteomes of these microorganisms can support the marine food web.
Experimental procedures
Bacterial strains and growth conditions
Marine Synechococcus strains BL107 (clade IV), CC9311 (clade I), RS9916 (clade IX), WH5701 (subcluster 5.2), WH7803 (clade V), WH7805 (clade VI) and WH8102 (clade III) were routinely grown in ASW medium (Wilson et al., 1996) at 22°C with a light intensity of 10 μmol photons m−2 s−1. Synechococcus sp. RS9917 was grown in ASW medium supplemented with 5 mM (NH4)2SO4 because this strain cannot utilize nitrate for growth (Fuller et al., 2003). Synechococcus spp. WH5701, WH7803, WH7805 and WH8102 are all axenic strains while the remaining strains are clonal but non‐axenic. Synechococcus sp. WH8102 was also grown (i) in co‐culture with the heterotrophic bacterium R. pomeroyi DSS‐3 (the latter at a density of 106 cells ml−1), (ii) in the dark for 10 h and (iii) in the presence of cyanophage S‐RSM4 (at a multiplicity of infection of 0.25) for 10 h. In this latter case, exoproteomes were prepared prior to cell lysis. Co‐cultures of Synechococcus sp. WH7803 with R. pomeroyi DSS‐3 were performed with similar starting cell concentrations. Synechococcus sp. WH7805 was similarly incubated in the dark.
Preparation of exoproteomes for nanoLC‐MS/MS analysis
Cultures for exoproteome analysis were prepared by spinning the cells (3000 g at room temperature during 15 min) and gently re‐suspending them in fresh media at a final concentration of 3 × 107 cell ml−1. Cultures were then left to grow in standard conditions to cell densities of 108 cells ml−1. At this point, cultures were subjected to centrifugation at 3000 g for 15 min at room temperature. Supernatants were carefully removed and then gently filtered through 0.22 μm pore size filter units (Sterivex‐GV, Millipore) to eliminate any remaining cells. Proteins in the remaining milieu were concentrated and purified by precipitation with trichloroacetic acid and run on SDS‐PAGE as previously described (Christie‐Oleza and Armengaud, 2010). Trypsin in‐gel proteolysis of the entire exoproteome was performed for the shotgun proteomics analysis as recommended (Hartmann et al., 2014). NanoLC‐MS/MS experiments were performed using a LTQ‐Orbitrap XL hybrid mass spectrometer (ThermoFisher) coupled to an UltiMate 3000 LC system (Dionex‐LC Packings). Conditions used were those previously described (de Groot et al., 2009).
MS/MS database search, abundance and comparative analysis
Compiled MS/MS spectra were searched against the annotated coding domain sequences of each strain downloaded from the NCBI (10/07/2012). Searches were carried out with MASCOT 2.2.04 software (Matrix Science) using parameters previously established (Christie‐Oleza et al., 2012). MASCOT results were parsed and peptides were filtered at a P‐value below 0.05. A protein was considered validated when at least two unique peptides were detected in the same experiment. A false‐positive rate below 0.1% for protein identification was estimated using a reverse decoy database as previously done (Christie‐Oleza et al., 2012). Protein quantification by spectral abundance was done as previously described (Liu et al., 2004). For normalized spectral abundance factors of each protein, spectral counts assigned to each polypeptide were divided by its molecular weight. Values were then normalized by the total sum corresponding to all the polypeptides detected with two or more non‐redundant peptides. Statistical comparisons between Synechococcus sp. WH8102 mass spectrometry‐detected exoproteins following growth under different conditions was carried out with the TFold method of the PatternLab program (Carvalho et al., 2012). A BH‐FDR statistical test was calculated to evaluate the global false discovery rate for each comparison.
Protein quantification and BSA‐degradation experiments
Protein was quantified with QuantiPro BCA Assay kit (Sigma‐Aldrich). Degradation of Bovine serum albumin BSA was used to determine the potential exoprotease activity of cultures. Experiments were performed in 48‐well plates with 800 μl culture (Synechococcus sp. WH7803 at cells ml−1 and R. pomeroyi DSS‐3 at 107 cells ml−1) and 200 μl 0.1% (w/v) BSA in ASW medium. ASW medium alone was used as a control. After culture incubation in standard conditions (see above), a 20 μl volume of supernatant was resolved and visualized by SDS‐PAGE as described in Christie‐Oleza and Armengaud (2010).
Protein in silico analysis
The theoretical exoproteome of the eight Synechococcus strains used in the experimental study plus four Prochlorococcus strains (MED4, MIT9303, MIT9312 and MIT9313) and SAR11 (Pelagibacter ubique HTCC1062) was determined based on three prediction tools: (i) the SignalP 4.0 server for predicting N‐terminal signal peptides for secretion (Petersen et al., 2011), (ii) the SecretomeP 2.0 server for predicting proteins exported by non‐classical systems (Bendtsen et al., 2005) and (iii) the LipoP server for predicting lipoproteins (Juncker et al., 2003). PSORTb 3.0 was used to determine subcellular location of the proteins (Yu et al., 2010). Local BLASTp analyses were done with the BioEdit BLAST tool v.7.0.5.3 (Hall, 1999) using default parameters and an E‐value cut‐off < 10−20. Conserved protein domains and motifs were determined using the Conserved Domains tool at the NCBI (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Supporting information
Table S1. Exported proteome prediction of eight Synechococcus strains, four Prochlorococcus strains and SAR11.
Table S2. Homology of each CDS with the closest CDS in the other 11 strains (plus SAR11) with tentative functions. The subset of giant proteins can be found in Table S2B.
Table S3. MS‐detected peptides from the exoproteomes of eight Synechococcus strains and list of polypeptides detected with two or more peptides.
Table S4. LC‐MS/MS detected proteins from the exoproteome of eight different Synechococcus strains grouped by abundance and homology.
Table S5. MS‐detected peptides from the exoproteome of Synechococcus WH8102 under different conditions and list of polypeptides detected with two or more peptides.
Table S6. Comparative proteomics of the exoproteomes of Synechococcus WH8102 submitted to different conditions.
Appendix S1. Supplementary data.
Acknowledgements
This work was supported by the FP7 Marie Curie Actions PIEF‐GA‐2010‐272593, the EU project MaCuMBA (Marine Microorganisms: Cultivation Methods for Improving their Biotechnological Applications; grant agreement no: 311975) to DJS, NERC Independent Research Fellowship NE/K009044/1 and the Commissariat à l'Energie Atomique et aux Energies Alternatives. The authors declare no conflict of interest.
References
- Armengaud, J. , Christie‐Oleza, J.A. , Clair, G. , Malard, V. , and Duport, C. (2012) Exoproteomics: exploring the world around biological systems. Expert Rev Proteomics 9: 561–575. [DOI] [PubMed] [Google Scholar]
- Avrani, S. , Wurtzel, O. , Sharon, I. , Sorek, R. , and Lindell, D. (2011) Genomic island variability facilitates Prochlorococcus‐virus coexistence. Nature 474: 604–608. [DOI] [PubMed] [Google Scholar]
- Barnett, J.P. , Robinson, C. , Scanlan, D.J. , and Blindauer, C.A. (2011) The Tat protein export pathway and its role in cyanobacterial metalloprotein biosynthesis. FEMS Microbiol Lett 325: 1–9. [DOI] [PubMed] [Google Scholar]
- Bendtsen, J.D. , Kiemer, L. , Fausboll, A. , and Brunak, S. (2005) Non‐classical protein secretion in bacteria. BMC Microbiol 5: 58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller, S.J. , Schubotz, F. , Roggensack, S.E. , Thompson, A.W. , Summons, R.E. , and Chisholm, S.W. (2014) Bacterial vesicles in marine ecosystems. Science 343: 183–186. [DOI] [PubMed] [Google Scholar]
- Brunner, E. , Richthammer, P. , Ehrlich, H. , Paasch, S. , Simon, P. , Ueberlein, S. , et al (2009) Chitin‐based organic networks: an integral part of cell wall biosilica in the diatom Thalassiosira pseudonana . Angew Chem Int Ed Engl 48: 9724–9727. [DOI] [PubMed] [Google Scholar]
- Carvalho, P.C. , Yates, J.R., III , and Barbosa, V.C. (2012) Improving the TFold test for differential shotgun proteomics. Bioinformatics 28: 1652–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie‐Oleza, J.A. , and Armengaud, J. (2010) In‐depth analysis of exoproteomes from marine bacteria by shotgun liquid chromatography‐tandem mass spectrometry: the Ruegeria pomeroyi DSS‐3 case‐study. Mar Drugs 8: 2223–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie‐Oleza, J.A. , Pina‐Villalonga, J.M. , Bosch, R. , Nogales, B. , and Armengaud, J. (2012) Comparative proteogenomics of twelve Roseobacter exoproteomes reveals different adaptive strategies among these marine bacteria. Mol Cell Proteomics 11: M111.013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, M.L. , Sullivan, M.B. , Martiny, A.C. , Steglich, C. , Barry, K. , Delong, E.F. , et al (2006) Genomic islands and the ecology and evolution of Prochlorococcus . Science 311: 1768–1770. [DOI] [PubMed] [Google Scholar]
- Cox, A.D. , and Saito, M.A. (2013) Proteomic responses of oceanic Synechococcus WH8102 to phosphate and zinc scarcity and cadmium additions. Front Microbiol 4: 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, H.‐P. , Wang, D.‐Z. , Xie, Z.‐X. , Dai, M.‐H. , and Hong, H.‐S. (2013) Metaproteomic characterization of high molecular weight dissolved organic matter in surface seawaters in the South China Sea. Geochim Cosmochim Acta 109: 51–61. [Google Scholar]
- Dufresne, A. , Ostrowski, M. , Scanlan, D.J. , Garczarek, L. , Mazard, S. , Palenik, B.P. , et al (2008) Unraveling the genomic mosaic of a ubiquitous genus of marine cyanobacteria. Genome Biol 9: R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, F.F. , Raftery, M.J. , Egan, S. , and Kjelleberg, S. (2007) Profiling the secretome of the marine bacterium Pseudoalteromonas tunicata using amine‐specific isobaric tagging (iTRAQ). J Proteome Res 6: 967–975. [DOI] [PubMed] [Google Scholar]
- Frischkorn, K.R. , Stojanovski, A. , and Paranjpye, R. (2013) Vibrio parahaemolyticus type IV pili mediate interactions with diatom‐derived chitin and point to an unexplored mechanism of environmental persistence. Environ Microbiol 15: 1416–1427. [DOI] [PubMed] [Google Scholar]
- Fuchs, S. , Grill, E. , Meskiene, I. , and Schweighofer, A. (2013) Type 2C protein phosphatases in plants. FEBS J 280: 681–693. [DOI] [PubMed] [Google Scholar]
- Fuller, N.J. , Marie, D. , Partensky, F. , Vaulot, D. , Post, A.F. , and Scanlan, D.J. (2003) Clade‐specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Appl Environ Microbiol 69: 2430–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooday, G.W. (1990) The ecology of chitin degradation In Advances in Microbial Ecology. Marshall K.C. (ed.). New York, USA: Springer, pp. 387–419. [Google Scholar]
- de Groot, A. , Dulermo, R. , Ortet, P. , Blanchard, L. , Guerin, P. , Fernandez, B. , et al (2009) Alliance of proteomics and genomics to unravel the specificities of Sahara bacterium Deinococcus deserti . PLoS Genet 5: e1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, T.A. (1999) BioEdit: a user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41: 95–98. [Google Scholar]
- Hartmann, E.M. , Allain, F. , Gaillard, J.C. , Pible, O. , and Armengaud, J. (2014) Taking the shortcut for high‐throughput shotgun proteomic analysis of bacteria. Methods Mol Biol 1197: 275–285. [DOI] [PubMed] [Google Scholar]
- Jardillier, L. , Zubkov, M.V. , Pearman, J. , and Scanlan, D.J. (2010) Significant CO2 fixation by small prymnesiophytes in the subtropical and tropical northeast Atlantic Ocean. ISME J 4: 1180–1192. [DOI] [PubMed] [Google Scholar]
- Johnson‐Rollings, A.S. , Wright, H. , Masciandaro, G. , Macci, C. , Doni, S. , Calvo‐Bado, L.A. , et al (2014) Exploring the functional soil‐microbe interface and exoenzymes through soil metaexoproteomics. ISME J 8: 2148–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncker, A.S. , Willenbrock, H. , Von Heijne, G. , Brunak, S. , Nielsen, H. , and Krogh, A. (2003) Prediction of lipoprotein signal peptides in Gram‐negative bacteria. Protein Sci 12: 1652–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashtan, N. , Roggensack, S.E. , Rodrigue, S. , Thompson, J.W. , Biller, S.J. , Coe, A. , et al (2014) Single‐cell genomics reveals hundreds of coexisting subpopulations in wild Prochlorococcus . Science 344: 416–420. [DOI] [PubMed] [Google Scholar]
- Kathuria, S. , and Martiny, A.C. (2011) Prevalence of a calcium‐based alkaline phosphatase associated with the marine cyanobacterium Prochlorococcus and other ocean bacteria. Environ Microbiol 13: 74–83. [DOI] [PubMed] [Google Scholar]
- Kettler, G.C. , Martiny, A.C. , Huang, K. , Zucker, J. , Coleman, M.L. , Rodrigue, S. , et al (2007) Patterns and implications of gene gain and loss in the evolution of Prochlorococcus . PLoS Genet 3: e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Sadygov, R.G. , and Yates, J.R., III (2004) A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem 76: 4193–4201. [DOI] [PubMed] [Google Scholar]
- McCarren, J. , and Brahamsha, B. (2007) SwmB, a 1.12‐megadalton protein that is required for nonflagellar swimming motility in Synechococcus . J Bacteriol 189: 1158–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh, B. , Dominguez‐Martin, M.A. , Gomez‐Baena, G. , Lopez‐Lozano, A. , Diez, J. , Barcena, J.A. , et al (2012) Nitrogen starvation induces extensive changes in the redox proteome of Prochlorococcus sp. strain SS120. Environ Microbiol Rep 4: 257–267. [DOI] [PubMed] [Google Scholar]
- Mackey, K.R. , Paytan, A. , Caldeira, K. , Grossman, A.R. , Moran, D. , McIlvin, M. , et al (2013) Effect of temperature on photosynthesis and growth in marine Synechococcus spp. Plant Physiol 163: 815–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary, I. , Tarran, G.A. , Warwick, P.E. , Terry, M.J. , Scanlan, D.J. , Burkill, P.H. , et al (2008) Light enhanced amino acid uptake by dominant bacterioplankton groups in surface waters of the Atlantic Ocean. FEMS Microbiol Ecol 63: 36–45. [DOI] [PubMed] [Google Scholar]
- Mazard, S. , Wilson, W.H. , and Scanlan, D.J. (2012) Dissecting the physiological response to phosphorus stress in marine Synechococcus isolates (Cyanophyceae). J Phycol 48: 94–105. [DOI] [PubMed] [Google Scholar]
- Montesinos, M.L. , Herrero, A. , and Flores, E. (1997) Amino acid transport in taxonomically diverse cyanobacteria and identification of two genes encoding elements of a neutral amino acid permease putatively involved in recapture of leaked hydrophobic amino acids. J Bacteriol 179: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, L.R. , Ostrowski, M. , Scanlan, D.J. , Feren, K. , and Sweetsir, T. (2005) Ecotypic variation in phosphorus‐acquisition mechanisms within marine picocyanobacteria. Aquat Microb Ecol 39: 257–269. [Google Scholar]
- Muñoz‐Marín, M.C. , Luque, I. , Zubkov, M.V. , Hill, P.G. , Diez, J. , and Garcia‐Fernandez, J.M. (2013) Prochlorococcus can use the Pro1404 transporter to take up glucose at nanomolar concentrations in the Atlantic Ocean. Proc Natl Acad Sci USA 110: 8597–8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenik, B. , Brahamsha, B. , Larimer, F.W. , Land, M. , Hauser, L. , Chain, P. , et al (2003) The genome of a motile marine Synechococcus . Nature 424: 1037–1042. [DOI] [PubMed] [Google Scholar]
- Pandhal, J. , Wright, P.C. , and Biggs, C.A. (2007) A quantitative proteomic analysis of light adaptation in a globally significant marine cyanobacterium Prochlorococcus marinus MED4. J Proteome Res 6: 996–1005. [DOI] [PubMed] [Google Scholar]
- Partensky, F. , Hess, W.R. , and Vaulot, D. (1999) Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev 63: 106–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz‐Yepes, J. , Brahamsha, B. , and Palenik, B. (2013) Role of a microcin‐C‐like biosynthetic gene cluster in allelopathic interactions in marine Synechococcus . Proc Natl Acad Sci USA 110: 12030–12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, T.N. , Brunak, S. , von Heijne, G. , and Nielsen, H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8: 785–786. [DOI] [PubMed] [Google Scholar]
- Saier, M.H., Jr (2006) Protein secretion and membrane insertion systems in gram‐negative bacteria. J Membr Biol 214: 75–90. [DOI] [PubMed] [Google Scholar]
- Scanlan, D.J. , Ostrowski, M. , Mazard, S. , Dufresne, A. , Garczarek, L. , Hess, W.R. , et al (2009) Ecological genomics of marine picocyanobacteria. Microbiol Mol Biol Rev 73: 249–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, D. (2014) Protein targeting, transport and translocation in cyanobacteria In The Cell Biology of Cyanobacteria. Flores E., and Herrero A. (eds). Seville, Spain: Caister Academic Press, pp. 121–147. [Google Scholar]
- Strom, S.L. , Brahamsha, B. , Fredrickson, K.A. , Apple, J.K. , and Rodriguez, A.G. (2012) A giant cell surface protein in Synechococcus WH8102 inhibits feeding by a dinoflagellate predator. Environ Microbiol 14: 807–816. [DOI] [PubMed] [Google Scholar]
- Stuart, R.K. , Brahamsha, B. , Busby, K. , and Palenik, B. (2013) Genomic island genes in a coastal marine Synechococcus strain confer enhanced tolerance to copper and oxidative stress. ISME J 7: 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztukowska, M. , Bugno, M. , Potempa, J. , Travis, J. , and Kurtz, D.M., Jr (2002) Role of rubrerythrin in the oxidative stress response of Porphyromonas gingivalis . Mol Microbiol 44: 479–488. [DOI] [PubMed] [Google Scholar]
- Tai, V. , Paulsen, I.T. , Philippy, K. , Johnson, D.A. , and Palenik, B. (2009) Whole‐genome microarray analyses of Synechococcus‐Vibrio interactions. Environ Microbiol 11: 2698–2709. [DOI] [PubMed] [Google Scholar]
- Waldbauer, J.R. , Rodrigue, S. , Coleman, M.L. , and Chisholm, S.W. (2012) Transcriptome and proteome dynamics of a light‐dark synchronized bacterial cell cycle. PLoS ONE 7: e43432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, W.H. , Carr, N.G. , and Mann, N.H. (1996) The effect of phosphate status on the kinetics of cyanophage infection in the oceanic cyanobacterium Synechococcus sp WH7803. J Phycol 32: 506–516. [Google Scholar]
- Yu, N.Y. , Wagner, J.R. , Laird, M.R. , Melli, G. , Rey, S. , Lo, R. , et al (2010) PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26: 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Yang, X. , Wang, H. , and van der Donk, W.A. (2014) High divergence of the precursor peptides in combinatorial lanthipeptide biosynthesis. ACS Chem Biol 9: 2686–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, A.E. , Martiny, A.C. , and Allison, S.D. (2013) Microdiversity of extracellular enzyme genes among sequenced prokaryotic genomes. ISME J 7: 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubkov, M.V. , Fuchs, B.M. , Tarran, G.A. , Burkill, P.H. , and Amann, R. (2003) High rate of uptake of organic nitrogen compounds by Prochlorococcus cyanobacteria as a key to their dominance in oligotrophic oceanic waters. Appl Environ Microbiol 69: 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Exported proteome prediction of eight Synechococcus strains, four Prochlorococcus strains and SAR11.
Table S2. Homology of each CDS with the closest CDS in the other 11 strains (plus SAR11) with tentative functions. The subset of giant proteins can be found in Table S2B.
Table S3. MS‐detected peptides from the exoproteomes of eight Synechococcus strains and list of polypeptides detected with two or more peptides.
Table S4. LC‐MS/MS detected proteins from the exoproteome of eight different Synechococcus strains grouped by abundance and homology.
Table S5. MS‐detected peptides from the exoproteome of Synechococcus WH8102 under different conditions and list of polypeptides detected with two or more peptides.
Table S6. Comparative proteomics of the exoproteomes of Synechococcus WH8102 submitted to different conditions.
Appendix S1. Supplementary data.


