Highlights
-
•
Mucinous adenocarcinoma associated with suppurative hidradenitis is extremely rare.
-
•
Magnetic resonance imaging findings help in the diagnosis of mucinous adenocarcinoma.
-
•
The surgery is highly complex, and even in a tertiary hospital, complications may occur.
Keywords: Mucinous adenocarcinoma, Chronic suppurative hidradenitis, Case report, Colorectal surgery
Abstract
Introduction
Chronic suppurative hidradenitis (CSH) is a benign condition that can affect the perineal region and often leads to the formation of abscesses and fistulas. It is rare for CSH to undergo malignant degeneration into mucinous adenocarcinoma.
Presentation of case
We report a case of a 55-year-old male patient with perineal CSH who suffered worsening long-term pain despite multiple surgical procedures to alleviate his symptoms. Pelvic magnetic resonance imaging (MRI) showed multiloculated cystic lesion on the left side wall of the distal rectum with gluteal extension. Pathological examination revealed mucinous adenocarcinoma. The patient underwent an abdominoperineal resection (APR) of the rectum with cutaneous muscle flap reconstruction. Although histopathological sections showed clear margins, the tumor recurred 6 months following surgery.
Discussion
Perineal mucinous adenocarcinoma arising in a patient with CSH is an extremely rare condition. This diagnosis is often difficult, due to the paucity of signs of malignant degeneration as well as the rarity of the disease itself. Surgical resection of the lesions is a well-established approach. In this case, diagnosing the tumor at such a late stage likely compromised his outcome.
Conclusion
Malignant degeneration to mucinous adenocarcinoma must be suspected in patients with a history of long-term CSH. In such cases, local biopsies and a radiological examination, such as MRI can help in the diagnosis.
1. Introduction
Chronic suppurative hidradenitis (CSH) is a condition where there is an infection of apocrine glands and surrounding soft tissues of the skin and adjacent structures. This often leads to the formation of fistulas and abscesses. When there is perianal involvement with extensive infection, there is an indication for wide resection of the affected area with primary or secondary closure. One of the rare complications is malignant degeneration, squamous cell carcinoma being the most common [1], [2], [3], [4], [5].
Mucinous adenocarcinoma in the perianal region is an uncommon condition and constitutes only 3% to 11% of all perianal cancers. This type of tumor can arise from anal glands and often presents itself as abscesses and/or fistulas, which compromises its early diagnosis [6], [7], [8]. The pathogenesis of those perianal tumors is still unknown and there are no histopathologic methods to demonstrate the primary association between the lesion and apocrine glands or anal glands. There are few reports in the literature of patients with perianal CSH who develop mucinous adenocarcinoma, making it an extremely rare association. Our aim here is to present a patient with perianal CSH who developed mucinous adenocarcinoma and to also review of the cases already published in the literature.
2. Presentation of the case
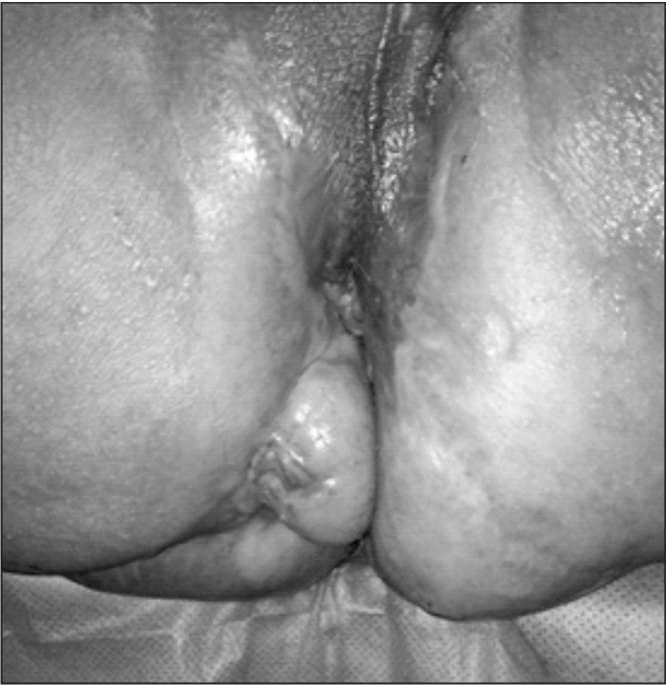
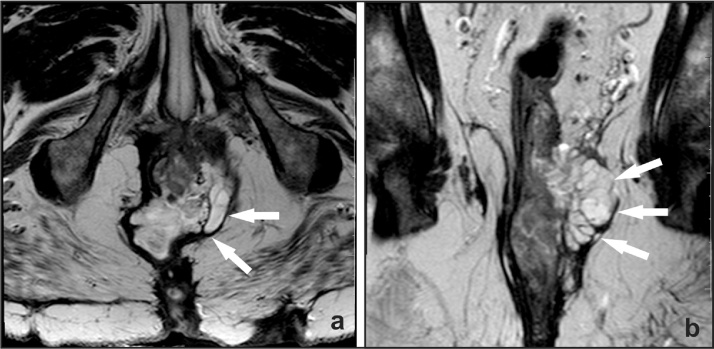
A 55-year-old male patient was followed at our Coloproctology Outpatient Clinic at the University of Campinas for the past 8 years. He was sent to our Clinic because of multiple perianal fistulas that began at 12 years of age requiring several surgical procedures (excision of the fistula tracks and abscess drainage). He was diagnosed early with CSH and throughout his treatment he reported progressive worsening of perineal and perianal pain, alteration in stool frequency, and mucinous discharge from his fistulas. Physical examination showed multiple deformities of the perianal region, scars, the presence of perineal skin ulcers near the posterior anal canal, mucinous discharge from fistulas, and a bulge in the perineal region (Fig. 1). Pelvic MRI revealed a multiloculated cystic lesion in the left side wall of the distal rectum, measuring 5.2 × 8.5 × 6.2 cm (Fig. 2).
Fig. 1.
Perineal Chronic Suppurative Hidradenitis, and scars from previous surgical resections. Patient in lithotomy position.
Fig. 2.
Magnetic Resonance Imaging shows hyper signal in T2, suggesting mucinous adenocarcinoma. (a) Transverse section (b) Sagittal section.
The lesion occupied the intersphincteric space with caudal extension bulging the elevator muscle of the anus bilaterally. There were fistulas between the lesion and the gluteal skin surface as well as the intergluteus groove with paths going both posteriorly and anteriorly to the presacral space bordering the anal elevator muscle in the right. There was a marked hyper signal in T2 and skin scar retraction.
The patient underwent an examination under analgesia with lesion biopsies. This revealed mucinous adenocarcinoma with a moderately differentiated pattern in the perianal region and associated inflammatory reactions near the epithelium. The skin had pseudoepitheliomatous hyperplasia and dermal fibrosis with the presence of 70% of mucinous component in the sample. Computed Tomography (CT) scan did not reveal distant metastasis.
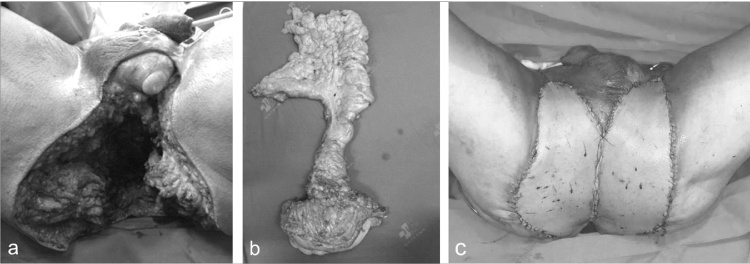
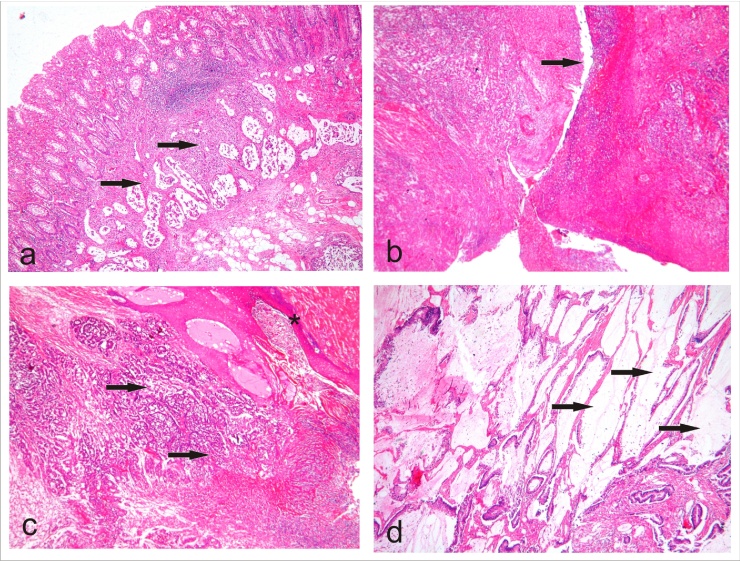
Given these findings, the patient elected to undergo an extended abdominoperineal resection (APR) with coccyx and partial sacrum resection, definitive colostomy, and pelvic lymphadenectomy. In the 12th post-operative day, a bilateral pedicled anterolateral thigh flap was performed by the Plastic Surgery team of the University of Campinas Clinical Hospital to cover the perineal defect. The surgical aspects are shown in Fig. 3. Unfortunately, he developed abdominal suture dehiscence with evisceration, making it necessary to perform a peritoneostomy. He also required cleaning of the perineal wound in the operating room and hyperbaric chamber therapy due to flap infection. He was discharged on post-operative day 60, in good condition and with good healing of the perineum. Histopathological sections confirmed a large mucinous adenocarcinoma with lymphatic and perineural invasion with clear margins and the absence of lymph node metastases (Fig. 4).
Fig. 3.
Abdominoperineal resection: (a) Surgical aspect after tumor removal (b) Surgical specimen (c) Perineal reconstruction with bilateral pedicled anterolateral thigh flaps.
Fig. 4.
Histological analysis: (a) Intact rectal mucosa, and presence of mucinous adenocarcinoma (arrows) in the submucosa, suggesting extra-rectal mucosa origin (b) Perineal fistula tract in CSH area (arrow), showing mucinous adenocarcinoma near the fistula and extensive area of necrosis (c) Mucinous adenocarcinoma (arrows) near the perineal skin (squamous epithelium*) (d)Tumor area with mucinous production (arrows).
Five months after surgery, he presented with a hardened and painful lesions in the previous flap in the perineal area. A new biopsy was performed confirming tumor recurrence, although the margins of the surgical specimen were clear. He elected not to pursue any other therapies due to the large extension of the lesion and his poor clinical condition. He died 6 months after the operation.
3. Discussion
Perineal mucinous adenocarcinoma is a rare clinical condition, and only 5 cases (4 male) associated with CSH were described in the literature (Table 1). They were between 40 and 60 years-old, and they had developed clinical presentation of CSH between the ages of 3 and 15 years. Ariwa [9] performed histochemical analysis which suggested that the origin was from the anal glands. In the present case report, this analysis was not performed for technical limitations. However, there were no significant alterations in the histologic sections of rectal mucosa from the surgical specimen, suggesting that the origin of the tumor may be associated with CSH. Indeed, the mucinous adenocarcinoma was found in the perineal and perianal subcutaneous tissue, near the fistulae tracks of the CSH. In all cases of the literature, malignant degeneration was diagnosed through lesion biopsies. The surgical approach was an extended APR in all patients with adjuvant radiotherapy in two cases and adjuvant chemotherapy and radiotherapy in another.
Table 1.
Mucinous adenocarcinoma in patients with Chronic Suppurative Hidradenitis (CSH).
| Reference | Patient | Clinical data | Treatment | Outcome |
|---|---|---|---|---|
| Ariwa [9] | Male 45 y.o. |
15 years of CSH. Biopsy of perianal ulcer revealed mucinous adenocarcinoma overlaid on anal squamous epithelium. | Abdominoperineal resection. | Recurrence and death four years and six months after surgery. |
| Bernard et al. [1] | Male 59 y.o. |
Gluteal and perineal suppuration for 11 months that began three years before being seen by the specialist. Extensive perianal lesions in the gluteal skin with the tumor in the middle of the lesions. |
Radical resection of the disease, near the anus and the levator anal muscle. Most of the gluteus maximus had to be excised to achieve safety margin. |
Two years later without disease, he gained weight, fecal continence preserved, and occasional loss of mucus caused by intermittent rectal mucosal prolapse. |
| Endo et al. [10] | Male 65 y.o. |
12 years of CSH. Diagnosis of tumor with extensive invasion. | NR | Died right after the diagnosis. |
| Marti et al. [11] | Male 39 y.o. |
The tumor was found during perianal abscess excision. | Abdominoperineal resection + adjuvant chemo and radiotherapy. | Follow-up of 1 year without recurrence. Died in the second year after the diagnosis. |
| do Val et al. [12] | Female 48 y.o. |
Extensive CSH in vulvar and perianal region for 10 years. | Abdominoperineal resection + adjuvant radiotherapy. | Follow-up of 1 year without recurrence. |
APR, abdominoperineal resection of the rectum; NR, not reported.
The most common symptoms of the mucinous adenocarcinoma are local pain (58%), rectal bleeding (40%) and perineal sense of mass (37%) [13]. In 80% of cases, the presentation of the initial lesion is usually more than 5 cm in diameter, which itself is associated with a poor prognosis. Detection is often delayed because these lesions mimic symptoms of benign anorectal inflammatory conditions and it can be difficult for biopsies to detect the tumor [11]. There is no consensus on the diagnosis and treatment of these tumors because of their rarity and the lack of controlled clinical trials. So far, the best treatment described is wide surgical resection. Radiotherapy with or without chemotherapy can be performed after surgery, but the results are controversial [5], [12], [14], [15], [16]. In the present report, the patient was not submitted to chemo and/or radiotherapy post-operatively due to wound infection and poor clinical condition.
The diagnosis is usually performed by biopsy of ulcerated lesions. None of the reported cases in the literature were diagnosed by MRI, even though it is the imaging method of choice, as it often reveals a characteristic hyper signal in T2 in the lesion area [17], [18], [19], [20]. Considering our case report, the diagnosis was strongly suspected with MRI and confirmed with incisional biopsy.
In all cases described in the literature, CSH coursed with abscesses and recurrent perianal fistulas. This reinforces the relationship between the perianal fistulas and the onset mucinous adenocarcinoma. In the present case, the diagnosis was suspected due to the significant worsening of the symptoms. It was not due to the clinical or digital rectal examination, as there were no significant changes in his perineal deformities from prior clinic visits. The majority of the reported cases, including the case currently described, died between 6 months and 4 years after surgery. This data suggests that mucinous adenocarcinoma associated with CSH has poor prognosis and shows the relevance of close follow-up for early detection of possible recurrences.
There is no consensus on how to follow these patients, since malignant degeneration is a rare association. However, it is well established that both complicated CSH and mucinous adenocarcinoma must be treated with a wide surgical resection of the lesions. Further studies are needed to understand the natural history of this association, so as to then create a diagnostic and intervention strategy.
4. Conclusion
Malignant degeneration of perineal CSH to mucinous adenocarcinoma is extremely rare. There are few reports in the literature and the purpose of this case report was to describe this malignant evolution of the CSH in which diagnosis was made primarily through MRI. The suspected diagnosis is difficult, both for the rarity of the disease and the poverty of specific signs and symptoms. In general, the surgical procedure to remove these lesions is highly complex, and complications may occur, especially when the lesion is diagnosed at an advanced stage.
Conflict of interest
Natália Mukai and other co-authors have no conflict of interest.
Funding
There was no funding for the research.
Ethical approval
The case report was performed in accordance with the Clinical Hospital Ethical Committee of the University of Campinas, number 004/2016.
Informed consent
The case report was performed in accordance with the Clinical Hospital Ethical Committee of the University of Campinas. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on requested. The case has been reported in line with the CARE criteria (http://www.care-statement.org/).
Author contribution
Natalia Mukhia contributed with data collection and wrote the paper.
Lílian Vital Pinheiro contributed with data collection and helped in the written of the manuscript.
Maria de Lourdes Setsuko Ayrizono contributed with data collection.
Guilherme Cardinali Barreiro contributed to the revision of the manuscript and participated in the plastic surgeries.
Paulo Kharmandayan contributed to the revision of the manuscript.
Mariana Hanayo Akinaga participated in the plastic surgeries.
Adriano Mesquita Bento participated in the plastic surgeries.
Carlos Augusto Real Martinez contributed with data collection.
Rita Barbosa de Carvalho contributed with surgical specimen pathological findings.
Marc Ward contributed with final version of the manuscript.
Cláudio Saddy Rodrigues Coy participated in the colorectal surgery.
Raquel Franco Leal participated in the colorectal surgery, contributed to the study design and revision of the manuscript.
Guarantor
Raquel Franco Leal, MD, PhD.
Acknowledgements
We thank Prof. T. Torriani of University of Campinas for an initial English review. We thank Dr. Marc Ward for his careful final review and editing of our manuscript.
References
- 1.Bernard B., Anderson C.A., Cadogan M. Hidradenitis suppurativa of the perineum, scrotum, and gluteal area: presentation, complications, and treatment. J. Natl. Med. Assoc. 1982;74(10):999–1003. [PMC free article] [PubMed] [Google Scholar]
- 2.Humphrey L.J., Playforth H., Leavell U.W., Jr. Squamous cell carcinoma arising in hidradenitis suppurativum. Arch. Dermatol. 1969;100:59–62. [PubMed] [Google Scholar]
- 3.Jackman R.J. Hidradenitis suppurativa. Diagnosis and surgical management of perianal manifestations. J. Proc. R. Soc. Med. 1959;52:110–112. [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson M.J., Dockerty M.B. Perianal hidradenitis suppurativa. A clinical and pathologic study. Dis. Colon Rectum. 1958;1:23–31. doi: 10.1007/BF02616510. [DOI] [PubMed] [Google Scholar]
- 5.Altunay K., Gökdemir G., Kurt A. Hidradenitis suppurativa and squamous cell carcinoma. Dermatol. Surg. 2002;28:88–90. doi: 10.1046/j.1524-4725.2002.01090.x. [DOI] [PubMed] [Google Scholar]
- 6.Patrinou V., Petrochilos J., Batistatou A. Mucinous adenocarcinoma arising in chronic perianal fistulas. J. Clin. Gastroenterol. 2001;33:175–176. doi: 10.1097/00004836-200108000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Getz S.B., Jr., Ough Y.D., Patterson R.B. Mucinous adenocarcinoma developing in chronic anal fistula: report of two cases and review of the literature. Dis. Colon Rectum. 1981;24:562–566. doi: 10.1007/BF02604325. [DOI] [PubMed] [Google Scholar]
- 8.Nelson R., Prasad M.L., Abcarian H. Anal carcinoma presenting as a perirectal abscess or fistula. Arch. Surg. 1985;120:632–635. doi: 10.1001/archsurg.1985.01390290106019. [DOI] [PubMed] [Google Scholar]
- 9.Ariwa R. Anal mucinous adenocarcinoma arising in perianal hidradenitis supprativa: a case report with histochemical study. Jpn. J. Proctol. 1980;33(6):581–586. [Google Scholar]
- 10.Endo Y., Tamura A., Ishikawa O. Perianal hidradenitis suppurativa: early surgical treatment gives good results in chronic or recurrent cases. Br. J. Dermatol. 1998;139:906–910. doi: 10.1046/j.1365-2133.1998.02524.x. [DOI] [PubMed] [Google Scholar]
- 11.Marti L., Nussbaumer P., Breitbach T. Perianal mucinous adenocarcinoma. A further reason for histological study of anal fistula or anorectal abscess. Chirurg. 2001;72(5):573–577. doi: 10.1007/s001040170137. [DOI] [PubMed] [Google Scholar]
- 12.do Val I.C., Almeida Filho G.L., Corrêa A. Chronic hidradenitis suppurativa and perianal mucinous adenocarcinoma. A case report. J. Reprod. Med. 2007;52(2):100–102. [PubMed] [Google Scholar]
- 13.Abel M.E., Chiu Y.S., Russell T.R. Adenocarcinoma of the anal glands. Results of a survey. Dis. Colon Rectum. 1993;36(4):383–387. doi: 10.1007/BF02053944. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi S., Yamanari H., Inada K. Adenocarcinoma in the anal canal associated with a fistula: report of a case. Surg. Today. 1996;26:707–710. doi: 10.1007/BF00312089. [DOI] [PubMed] [Google Scholar]
- 15.Anthony T., Simmang C., Lee E.L. Perianal mucinous adenocarcinoma. J. Surg. Oncol. 1997;64:218–221. doi: 10.1002/(sici)1096-9098(199703)64:3<218::aid-jso8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Schaffzin D.M., Stah T.J., Smith L.E. Perianal mucinous adenocarcinoma: unusual case presentations and review of the literature. Am. Surg. 2003;69:166–169. [PubMed] [Google Scholar]
- 17.Kim M.J., Park J.S., Park S.I. Accuracy in differentiation of mucinous and nonmucinous rectal carcinoma on MR imaging. J. Comput. Assist. Tomogr. 2003;27:48–55. doi: 10.1097/00004728-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Hussain S.M., Outwater E.K., Siegelman E.S. MR imaging features of pelvic mucinous carcinomas. Eur. Radiol. 2000;10:885–891. doi: 10.1007/s003300051029. [DOI] [PubMed] [Google Scholar]
- 19.Bocchini S.F., Habr-Gama A., Kiss D.R. Gluteal and perianal hidradenitis suppurativa. Surgical treatment by wide excision. Dis. Colon Rectum. 2003;46(7):944–949. doi: 10.1007/s10350-004-6691-1. [DOI] [PubMed] [Google Scholar]
- 20.Leal R.F., Ayrizono M.L.S., Coy C.S.R. Mucinous adenocarcinoma derived from chronic perianal fistulas: report of a case and review of the literature. Tech. Coloproctol. 2007;11:155–157. doi: 10.1007/s10151-007-0348-8. [DOI] [PubMed] [Google Scholar]