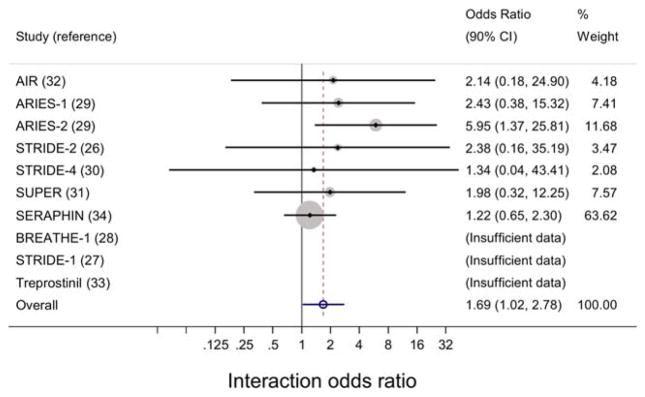
Figure 2.
Interaction effect estimates from individual studies and from meta-analysis of pooled studies to assess the impact of diagnosis-by-treatment interaction on adverse events in patients with connective tissue disease–associated pulmonary arterial hypertension and those with idiopathic pulmonary arterial hypertension. Forest plots show the odds ratios with 90% confidence intervals (90% CIs) for the diagnosis-by-treatment interactions and occurrence of adverse events. Three studies (the BREATHE-1 [Bosentan: Randomized Trial of Endothelin Receptor Antagonist Therapy for Pulmonary Arterial Hypertension 1], STRIDE-1 [Sitaxsentan to Relieve Impaired Exercise 1], and treprostinil studies) had an insufficient number of events and therefore could not be analyzed (I2 = 0.0%, P = 0.82). AIR = Aerosolized Iloprost Randomized Study; ARIES-1 = Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Study 1; SUPER = Sildenafil Use in Pulmonary Arterial Hypertension; SERAPHIN = Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.39220/abstract.