Figure 7.
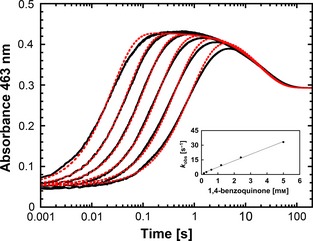
Oxidative half‐reaction of pyranose dehydrogenase from A. meleagris followed by stopped‐flow spectroscopy after mixing with BQ. A solution of fully reduced enzyme (30 μm) was mixed with buffer containing various BQ concentrations (0.15, 0.3, 0.6, 1.2, 2.4 and 5 mm in 50 mm potassium phosphate buffer, pH 7.0) at 4 °C under anaerobic conditions. All given concentrations are reported after mixing. The reaction was monitored at 463 nm. The kinetic traces from right to left correspond to increasing BQ concentrations. To evaluate the experimental traces (black), a double‐exponential fit was used. The first phase k obs(1) was the FAD oxidation with a large absorbance increase at 463 nm, which was used to calculate k app,4 °C. The experimental traces were simulated with berkeley madonna, version 8.3.14, according to Kinetic Model 1ox (red traces) with the parameter sets listed in Table 5. The inset shows the plot of the pseudo first‐order rate constants k obs(1) versus the BQ concentrations.
