Abstract
Protease-activated receptors have been shown to regulate endothelial nitric oxide synthase through the phosphorylation of specific sites on the enzyme. It has been established that PAR-2 activation phosphorylates eNOS-Ser-1177 and leads to the production of the potent vasodilator nitric oxide, while PAR-1 activation phosphorylates eNOS-Thr-495 and decreases nitric oxide production in human umbilical vein endothelial cells. In this study, we hypothesize a differential coupling of protease-activated receptors to the signaling pathways that regulates endothelial nitric oxide synthase and nitric oxide production in primary adult human coronary artery endothelial cells. Using Western Blot analysis, we showed that thrombin and the PAR-1 activating peptide, TFLLR, lead to the phosphorylation of eNOS-Ser-1177 in human coronary artery endothelial cells, which was blocked by SCH-79797 (SCH), a PAR-1 inhibitor. Using the nitrate/nitrite assay, we also demonstrated that the thrombin- and TFLLR-induced production of nitric oxide was inhibited by SCH and L-NAME, a NOS inhibitor. In addition, we observed that TFLLR, unlike thrombin, significantly phosphorylated eNOS-Thr-495, which may explain the observed delay in nitric oxide production in comparison to that of thrombin. Activation of PAR-2 by SLIGRL, a PAR-2 specific ligand, leads to dual phosphorylation of both catalytic sites but primarily regulated eNOS-Thr-495 phosphorylation with no change in nitric oxide production in human coronary artery endothelial cells. PAR-3, known as the non-signaling receptor, was activated by TFRGAP, a PAR-3 mimicking peptide, and significantly induced the phosphorylation of eNOS-Thr-495 with minimal phosphorylation of eNOS-Ser-1177 with no change in nitric oxide production. In addition, we confirmed that PAR-mediated eNOS-Ser-1177 phosphorylation was Ca2+-dependent using the Ca2+ chelator, BAPTA, while eNOS-Thr-495 phosphorylation was mediated via Rho kinase using the ROCK inhibitor, Y-27632, suggesting protease-activated receptor coupling to Gq and G12/13, respectively. These data suggest a vascular bed specific differential coupling of protease-activated receptors to the signaling pathways that regulate endothelial nitric oxide synthase and nitric oxide production that may be responsible for endothelial dysfunction associated with cardiovascular disease.
Keywords: Endothelial nitric-oxide synthase, nitric oxide, endothelial dysfunction, protease-activated receptors, thrombin
Introduction
Endothelial dysfunction is the primary predictor of systemic hypertension, its persistence contributing to severe cardiovascular events.1,2 Some of the current therapies include the manipulation of agonist/antagonist properties3 of protease-activated receptors (PARs), which are a family of G protein-coupled receptors that are primarily expressed in cells of the vasculature and known for their involvement in regulation of vascular tone. These receptors induce endothelium-dependent relaxation via production and release of a potent vasodilator, nitric oxide (NO), and therefore, play an important role in endothelial function.4,5 There are four members of the PAR family, PAR-1 through -4, that are uniquely irreversibly activated via cleavage by serine proteases that expose a neo-N-terminal, which acts as a self-activating peptide.5–7 PAR-1, -3, and -4, are directly activated by thrombin, a serine protease responsible for platelet aggregation, endothelial cell activation, and other important responses in vascular biology.4–6,8 Whereas, PAR-2 is the receptor for trypsin-like enzymes and can respond to thrombin only through transactivation by PAR-1.5,6 Activation of PARs via thrombin has been highly defined by its response to coagulant proteases but also stimulates an array of signal transduction pathways, which include the activation of distinct heterotrimeric G proteins (Gi, Go, Gq, and G12/13) and tyrosine and serine/threonine kinases.5,7 Accordingly, these signaling pathways are essential in the maintenance of vascular homeostasis.8–11
Recent advances in the cardiovascular characterization of PAR signaling make them extremely attractive for drug targeting3; however, how PARs regulate the human eNOS/NO pathway remains unclear. Studies have shown that multiple PARs modulate the production of NO via post-translational modifications of endothelial nitric oxide synthases (eNOS) via specific phosphorylation of various regulatory sites.6,7,12–14 Most of what is known regarding this regulation centers on the functional consequences of the phosphorylation of eNOS-Ser-1177/-1179 (human/bovine) and eNOS-Thr-495/-497 (human/bovine). When eNOS-Ser-1177/-1179 is phosphorylated, there is an increase in eNOS activity followed by an immediate increase in the production of NO. In contrast, when eNOS-Thr-495/-497 is phosphorylated, eNOS activity is down-regulated, which leads to a decrease in NO production.9,12,13,15
We have previously demonstrated the reciprocal regulation of the phosphorylation of these two sites on eNOS by PARs using bovine aortic endothelial cells (BAECs) and human umbilical vein endothelial cells (HUVECs). The two eNOS sites are phosphorylated by PARs via different G protein-dependent signal transduction pathways. Thrombin and a selective PAR-1 activating peptide (AP), TFLLR, modulate eNOS negatively by phosphorylating eNOS at the Thr-495 site and signaling through a Rho/ROCK-dependent pathway.12 Activation of PAR-2 via PAR-1 transactivation or using an activating peptide specific for PAR-2, SLIGRL, phosphorylates eNOS at Ser1177 through a Ca2+ and PKC-δ-sensitive, but PI3K/AKT-independent pathway.12,15 However, the role the highly co-expressed PAR-3 plays on eNOS phosphorylation still remains unknown, while PAR-4 has been shown to induce the production of NO in HUVECs.16 Alternatively, studies have shown that low amounts of shear stress increase ERK-mediated eNOS-Thr-495 phosphorylation to enhance vasoconstriction, which was reduced in the presence of the common phenol, resveratrol.17 While the deletion of Δ594–604 residues of the autoinhibitory element (AIE) within the FMN-binding domain of human eNOS regulates the Ca2+/calmodulin-dependent domain of eNOS to produce NO via phosphorylation of eNOS-Ser-1177 and dephosphorylation of eNOS-Thr-495.18,19
Here, we provide evidence of a deviation from the classical role of PARs in mediating the regulation of eNOS phosphorylation in humans, which is different from that observed previously in HUVECs.12,13 In this study, primary human coronary artery endothelial cells (HCAECs) were used as a model system to characterize the signaling potential of PARs in the critical vasculature altered in ischemic heart disease. Thrombin was observed to primarily regulate the positive site of eNOS, inducing phosphorylation of eNOS-Ser-1177. Activation of PAR-1 and PAR-2 using specific activating peptide ligands phosphorylated both regulatory sites with evidence of dual signaling pathways. However, only PAR-1 activation resulted in an increase in NO metabolites, which favors vasodilation, while selective PAR-2 activation prevented NO production, which favors vasoconstriction. In addition, we were able to characterize the functional role of PAR-3, which induced eNOS-Thr-495 phosphorylation only whereas PAR-4 was not expressed in these cells. These findings characterizing PAR-mediated eNOS/NO signaling in relevant HACECs will provide a greater understanding of vascular signaling events that will assist in the development of more specific therapeutic agents for the prevention and treatment of cardiovascular diseases.
Materials and methods
Materials
Thrombin (bovine derived), β-actin antibody, proteinase inhibitor cocktail, and phenylmethylsulfonyl fluoride (PMSF) were purchased from Sigma, St. Louis, MO. TFLLR and SCH-79797 were purchased from Tocris, Minneapolis, MN. TRAGAP was purchased from Bachem, Torrance, CA. L-NG-Nitroarginine methyl ester (L-NAME) and SCH-79797 were purchased from Cayman Chemical, Ann Arbor, MI. PAR-1, PAR-3 H-108,-2,-4 primary antibodies were purchased from Santa Cruz, Dallas, TX. eNOS-III, p-eNOS-Thr-495, p-eNOS-Ser-1177 were purchased from BD Bioscience, San Jose, CA. Primary antibodies, 1,2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid tetraacetoxy-methyl ester (BAPTA-AM), and ROCK inhibitor Y-27632 were purchased from Tocris, Minneapolis, MN.
Cell culture
Primary HCAECs from adult male and female (age 24-59) donors were purchased and used under the manufacturer’s (PromoCell, Heidelberg, Germany) instructions. Male HCAECs were also purchased from American Type Culture Collection (ATCC), Manassas, VA, and maintained the same as the cells from PromoCell. Cells were grown in endothelial cell growth medium (ECGM) macro vascular (MV) containing fetal calf serum, endothelial growth supplement, human recombinant epidermal growth factor, heparin, and hydrocortisone. At 80–90% confluency, the cells were subcultured using a trypsin-EDTA solution and 1 × phosphate-buffered saline (PBS). Experiments were performed in 6/12-well plates using confluent cells from passages 3–7, which were serum deprived in ECGM without MV for 12–18 h before each experiment to ensure a quiescent cell state.
Western blotting
Total protein was extracted from cells via lysing in ice-cold modified RIPA buffer with 1 mM Na3VO4, 20 mM β-glycerophosphate, sodium deoxycholate, with 1 mM PMSF, and 1% (v/v) protease inhibitor cocktail solution (Sigma, St. Louis, MO) freshly added. Up to 50 µg of protein from cell lysates was separated on 7.5% or 4–12% SDS-polyacrylamide and electrophoretically transferred to nitrocellulose membranes (BioRad, Hercules, CA). Blots were blocked with 5% (w/v) casein in TBS containing 0.1% (v/v) tween 20 and then incubated with the appropriate primary antibodies (phospho-specific antibodies for eNOS-Thr-495 and eNOS-Ser-1177 were used at a 1:1000 concentration, control antibodies, total eNOS, and β-actin, were used at 1:1000, 1:4000, and 1:5000, respectively) followed by horseradish peroxidase-labeled secondary antibody, and the bands were visualized by enhanced chemiluminescence (Pierce/Thermo Scientific, Rockford, IL).
Nitrate/nitrite (NO measurement)
Total NO was measured via total nitrate/nitrite (colorimetric and flurometric) assay (Cayman Chemical, Ann Arbor, MI), which measured nitrate/nitrite (NO2−/NO3−) production, an indicator of NO synthesis. Detection of nitrate/nitrite production is based on colorimetric detection of nitrite as an azo dye product of the Griess reaction. Nitrate/nitrite concentrations were calculated by comparison with the optical density 540 of standard solutions as directed by the manufacturer’s instructions. In brief, nitrate is converted into nitrite using nitrite reductase. The second step is the addition of the Griess reagents, which converts nitrite into a deep purple azo compound whose photometric measurement of the absorbance at 540 nm wavelength correlates total nitrite concentrations. To determine thrombin/AP-induced NO2−/NO3− accumulation, basal NO2−/NO3− accumulation was subtracted from values obtained with thrombin/AP stimulation conditions.
Statistics
The data presented in this study were representative of three or more separate experiments yielding similar results. Intensity of the bands was measured by UN-SCAN-IT gel automated digitizing system version 6.1 (Silk Scientific, Orem, UT) and/or image studio lite 3.1.4 software from (LI-COR Biosciences, Lincoln, NE). Data analysis was done with prism 6.0 statistical program software using student t-test and/or one-way ANOVA and expressed as standard error of the mean.
Results
Effects of thrombin on eNOS phosphorylation and NO production in HCAECs
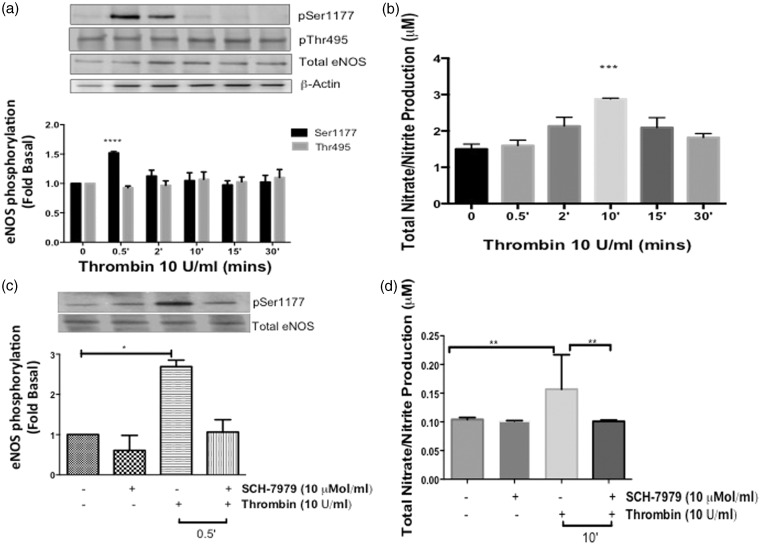
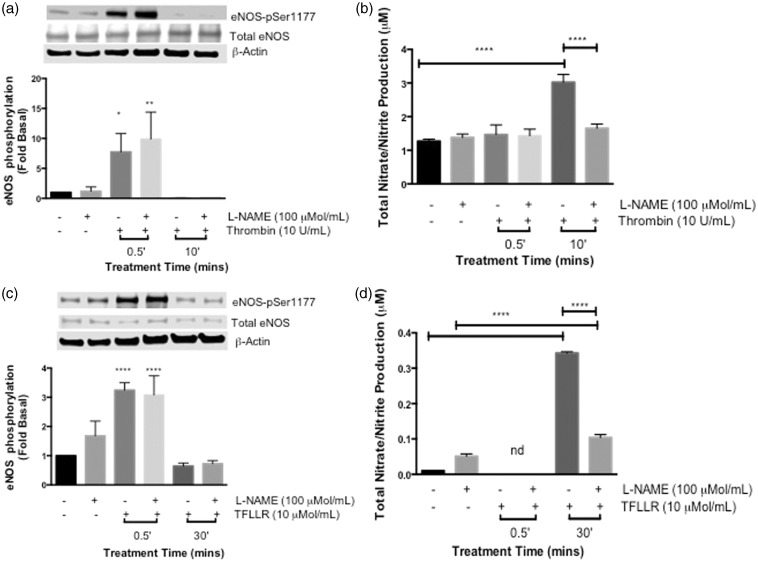
We previously demonstrated that thrombin induces post-translational modification of both eNOS-regulatory sites in a time-dependent manner to regulate NO production.12,13,15 To determine the physiological effects of thrombin in the adult coronary endothelium, HCAECs were stimulated with thrombin in a time-dependent manner from 30 s up to 30 min. The phosphorylation of eNOS at both regulatory sites was measured via Western blot. As shown in Figure 1(a), thrombin (10 U/ml) significantly stimulated phosphorylation at eNOS-Ser-1177 at the 30-s time point (P < 0.0001 vs. Basal), and occasional phosphorylation was detected at 2 min, which was not statistically significant. Phosphorylation at the later time points was comparable to basal levels. Thrombin did not stimulate an increase in the phosphorylation of eNOS-Thr-495 site. Using a colorimetric or fluorometric absorbance assay to measure eNOS enzymatic activity, total NO metabolites, nitrate/nitrite, were increased maximally at 10 min (P < 0.05 vs. Basal) in Figure 1(b).
Figure 1.
Effects of thrombin on eNOS phosphorylation and NO production in HCAECs (a) HCAECs were stimulated with thrombin (10 U/ml) for the indicated time points and phosphorylated eNOS-ser1177 and eNOS-Thr495 were determined using Western blot analysis with phosphospecific antibodies. Representative immunoblots are shown with densitometric analyses. Mean +/− SEM of measurements in cultures of five different passages; ****P < 0.0001 Thrombin 0.5 min versus Basal. (b) NO production was determined indirectly by measuring total nitrate/nitrite using media from HCAECs treated with thrombin (10 U/ml) for the indicated time points. n = 5, ***P < 0.05 Thrombin versus Basal. (c) Cells were pretreated with SCH-79797 (10 µmol/L) or vehicle (0.01% DMSO) for 1 h prior to stimulation with thrombin (10 U/ml) for 0.5 min. Phosphorylated eNOS-Ser1177 and eNOS-Thr495 were determined using Western blot analysis with phosphospecific antibodies. Representative immunoblots are shown with densitometric analyses. Mean +/− SEM of measurements in cultures of four different passages; *P < 0.001 Thrombin versus Vehicle. (d) Total nitrate/nitrite production was measured using media from HCAECs pretreated with SCH-79797 (10 µmol/L) or vehicle (0.01% DMSO) for 1 h prior to stimulation with thrombin (10 U/ml) for 10 min n = 3, **P < 0.05 Thrombin versus Basal and SCH + Thrombin 10 min
To confirm that the thrombin-eNOS-Ser-1177-NO response observed in Figure 1(a) and (b), was mediated via PAR-1 activation, we pretreated the HCAECs with a selective PAR-1 inhibitor, SCH-79797 (SCH) (10 µmol/ml) for 1 h prior to stimulation with thrombin (10 U/ml). As shown in Figure 1(c), SCH significantly inhibited the phosphorylation of eNOS-Ser-1177 (P < 0.001 vs. Vehicle); however, it had no effect on the basal levels of eNOS-Thr-495 phosphorylation observed by stimulation with thrombin (data not shown). In addition, SCH also inhibited the thrombin-induced production of NO metabolites at 10 min (P < 0.05) as shown in Figure 1(d). These findings demonstrate that thrombin induces the phosphorylation of eNOS-Ser-1177 site preferentially with an increase in NO production via PAR-1 activation in HCAECs.
Effects of PAR-1 activating peptide on eNOS phosphorylation and NO production in HCAECs
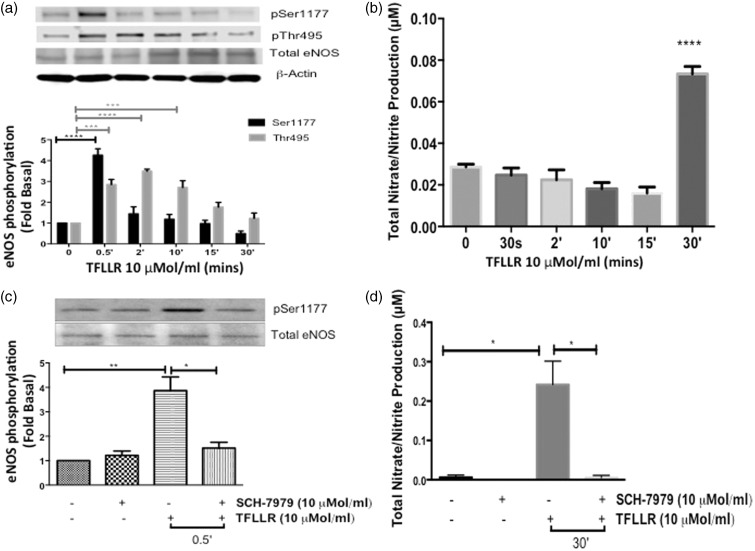
Thrombin most notably is the primary endogenous agonist for PAR-1, -3, and -4.5,20 To directly assess the effect of PAR-1 activation on eNOS regulation, we stimulated cells with a selective PAR-1-AP, TFLLR (10 µmol/ml) in a time-dependent manner, and measured the phosphorylation of the two eNOS regulatory sites in HCAECs. Similar to thrombin, TFLLR significantly phosphorylated eNOS-Ser-1177 at the 30-s time point (P < 0.0001) (Figure 2(a)). Unlike thrombin, TFLLR significantly phosphorylated eNOS-Thr-495 starting at 30 s and the phosphorylation remained constant up to 10 min (P < 0.001). Nitrate/nitrite was also measured following TFLLR stimulation and there was a significant increase at the 30-min time point, (P < 0.0001) (Figure 2(b)), which confirms PAR-1 mediated NO production. The delay in NO production observed when the cells were stimulated with TFLLR may be due to the input of eNOS-Thr-495. We observed that the phosphorylation of this catalytic site declines over time, and therefore, its negative affect may be eventually over-ridden by the earlier activation of eNOS-Ser-1177.
Figure 2.
Effects of PAR-1 activating peptide on eNOS phosphorylation and NO production in HCAECs (a) HCAECs were stimulated with TFLLR (10 µmol/L) for the indicated time points and phosphorylated eNOS-Ser1177 and eNOS-Thr495 were determined using Western blot analysis with phosphospecific antibodies ****P < 0.0001 TFLLR 0.5 min versus Basal, ***P < 0.001 TFLLR Thr495p 0.5 min, 2 min and 10 min versus Basal. (b) NO production was determined indirectly by measuring total nitrate/nitrite using media from HCAECs treated with TFLLR (10 µmol/L) for the indicated time points n = 3, ****P < 0.0001 TFLLR 30 min versus Basal. (c) Cells were pretreated with SCH-79797 (10 µmol/L) or vehicle (0.01% DMSO) for 1 hour prior to stimulation with TFLLR (10 µmol/L) for 0.5 min. Phosphorylated eNOS-Ser1177 was determined using Western blot analysis with phosphospecific antibody. Representative immunoblots are shown with densitometric analyses. Mean +/− SEM of measurements in cultures of six different passages; **P < 0.001 TFLLR 0.5 min versus Vehicle, *P < 0.005 TFLLR versus SCH + TFLLR 0.5 min. (d) Total nitrate/nitrite production was measured using media from HCAECs pretreated with SCH-79797 (10 µmol/L) or vehicle (0.01% DMSO) for 1 hour prior to stimulation with TFLLR (10 µmol/ml) for 30 min n = 3, *P < 0.01 TFLLR 30 min versus Vehicle; TFLLR 30 min versus SCH + TFLLR 30 min
To validate PAR-1 regulation of eNOS in HCAECs, we pretreated the cells with SCH (10 µmol/ml) for 1 h, which blocked TFLLR-mediated eNOS-Ser-1177 phosphorylation at 30 s (P < 0.001) and subsequent nitrate/nitrite production at 30 min (P < 0.01) (Figure 2(c) and (d). In our previous studies, we demonstrated that activation of PAR-1 by TFLLR in HUVECs leads to the phosphorylation of eNOS-Thr-495 and a decrease in NO production (29). These findings support the cell-type specific regulation of eNOS by PARs.
Effects of PAR-2 activating peptide on eNOS phosphorylation and NO production in HCAECs
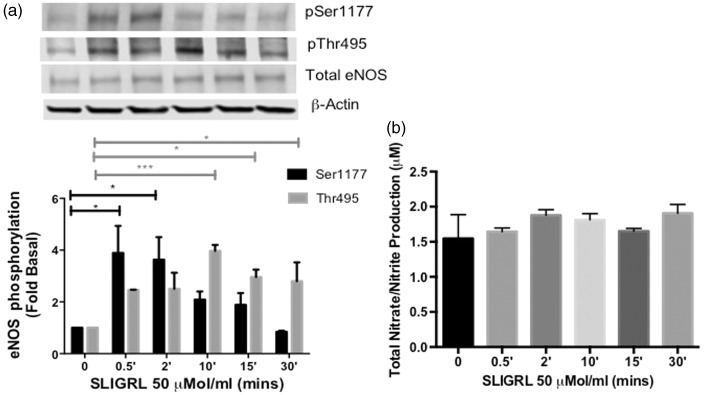
Thrombin has been characterized to phosphorylate both regulatory sites of eNOS via direct activation of PAR-1 and PAR-1 transactivation of PAR-2 in HUVECs.12,13,15 Because thrombin was shown to preferentially modulate PAR-1 activity in HCAECs, we wanted to investigate the role of PAR-2 in this cell line. Therefore, we used a PAR-2-AP, SLIGRL, to test direct PAR-2 function in the regulation of eNOS. HCAECs were stimulated with SLIGRL (50 µmol/ml) in a time-dependent manner, which led to significant phosphorylation of eNOS-Thr-495 at 10 (P < 0.0001), 15 (P < 0.05), and 30 min (P < 0.05) (Figure 3(a)). We also observed a significant increase in phosphorylation at eNOS-Ser-1177 at 30 s and 2 min (P < 0.05 vs. Basal). Consequently, total NO metabolites were not detected during the time-course when compared to basal levels (Figure 3(b)). These data suggest that PAR-2 can modulate both regulatory sites; however, activated PAR-2 sustained the phosphorylation of eNOS-Thr-495, demonstrating for the first time a functional dysregulation of PAR-2 signaling in HCAECs.
Figure 3.
Effects of PAR-2 activating peptide on eNOS phosphorylation and NO production in HCAECs (a) HCAECs were stimulated with SLIGRL (50 µmol/L) for the indicated time points and phosphorylated eNOS-ser1177 and eNOS-Thr495 was determined using Western blot analysis with phosphospecific antibodies. Representative immunoblots are shown with densitometric analyses. Mean +/− SEM of measurements in cultures of six different passages; ***P < 0 .001 SLIGRL Thr495p 10 min versus Basal, *P < 0.05 SLIGRL 15 min and 30 min versus Basal, *P < 0.05 SLIGRL Ser1177p 0.5 min and 2 min versus Basal. (b) NO production was determined indirectly by measuring total nitrate/nitrite using media from HCAECs treated with SLIGRL (50 µmol/L) for the indicated time points, n = 6, NS
Effects of PAR-3 activating peptide on eNOS phosphorylation and NO production in HCAECs
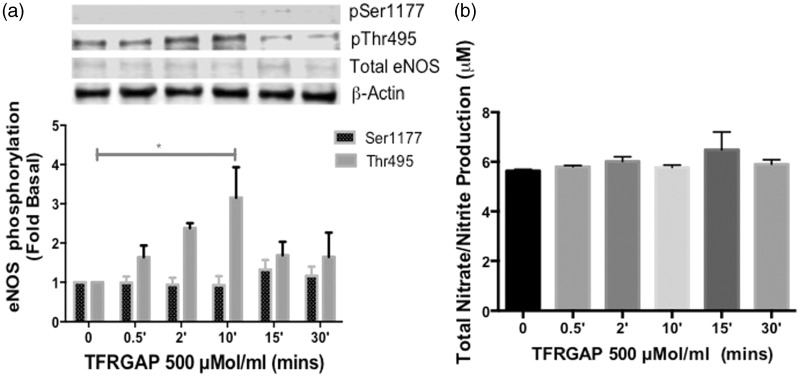
PAR-3 is classified as the second thrombin receptor, which is richly expressed in a wide variety of vascular cells, and is thought to possess no independent signaling capacity.5,20–22 In order to assess the function of PAR-3 in human endothelial cells, we stimulated the cells with a synthetic PAR-3-AP, TFRGAP (500 µmol/mL)23 in a time-dependent manner. Maximum phosphorylation was observed at 10 min (P < 0.05) at the negative regulatory site, eNOS-Thr-495. The positive site, eNOS-Ser-1177 showed no significant change in phosphorylation in comparison to basal (Figure 4(a)). In addition, there was no increase in NO metabolites above the basal level during the time-dependent stimulation with TFRGAP (Figure 4(b)). These data establish for the first time that PAR-3 primarily signals to regulate eNOS-Thr-495 in primary adult HCAECs.
Figure 4.
Effects of PAR-3 activating peptide on eNOS phosphorylation and NO production in HCAECs (a) HCAECs were stimulated with TFRGAP (500 µmol/L) for the indicated time points and phosphorylated eNOS-Ser1177 and eNOS-Thr495 was determined using Western blot analysis with phosphospecific antibodies. Representative immunoblots are shown with densitometric analyses. Mean +/− SEM of measurements in cultures of four different passages; *P < 0.05 TFRGAP Thr495p 10 min versus Basal. (b) NO production was determined indirectly by measuring total nitrate/nitrite using media from HCAECs treated with TFRGAP (500 µmol/L) for the indicated time points, n = 4, NS
Effects of NOS inhibitor, L-NAME, on PAR-1 mediated NO production in HCAECs
We previously reported that a NOS inhibitor blocked the thrombin-mediated regulation of eNOS/cGMP in bovine endothelial cells.15 Interestingly, the timing between thrombin- and TFLLR-induced eNOS-Ser-1177 phosphorylation and subsequent NO production in HCAECs is substantially delayed when compared to that observed in our previous studies, where PAR-mediated NO production occurred immediately after eNOS-Ser-1177 phosphorylation.12,13,15 Therefore, to confirm the role of PAR-1 activation in eNOS function, HCAECs were pretreated with eNOS inhibitor, L-NAME (100 µmol/L) 1 h prior to stimulation with thrombin or TFLLR. In Figure 5(a), thrombin caused a significant increase in eNOS-Ser-1177 phosphorylation (P < 0.05), which was not inhibited by L-NAME (P < 0.01) at the 30-s time point. However, at the 10-min time point eNOS-Ser-1177 phosphorylation was undetectable under the same treatment conditions. Consequently, L-NAME did not alter the basal level of NO metabolites when the cells were stimulated with thrombin for 30 s. However, a significant increase in NO metabolites was observed when the cells were stimulated with thrombin for 10 min (P < 0.001), and this increase in NO metabolites was significantly inhibited by L-NAME (P < 0.001) (Figure 5(b)).
Figure 5.
Effects of NOS inhibitor, L-NAME, on PAR-1 mediated NO production in HCAECs. (a) HCAECs were pretreated with L-NAME (100 µmol/L) for 1 h prior to stimulation with thrombin (10 U/ml) for 0.5 min and 10 min. Phosphorylation of eNOS-Ser1177 was determined using Western blot analysis with phosphospecific antibody. Representative immunoblots are shown with densitometric analyses. Mean +/− SEM of measurements in cultures three of different passages; *P < 0.05 Thrombin 0.5 min versus Basal, **P < 0.01 L-NAME + Thrombin 0.5 min versus Basal. (b) NO production was determined indirectly by measuring total nitrate/nitrite using media from HCAECs pretreated with L-NAME (100 µmol/L) for 1 h prior to stimulation with thrombin (10 U/ml) for 0.5 min and 10 min ****P < 0.001 Thrombin 0.5 min versus Basal, ****P < 0.001 L-NAME + Thrombin 0.5 min versus Basal. (c) HCAECs were pretreated with L-NAME (100 µmol/L) for 1 h prior to stimulation with TFLLR (10 µmol/ml) for 0.5 min and 10 min. Phosphorylation of eNOS-Ser1177 was determined using Western blot analysis with phosphospecific antibody. Representative immunoblots are shown with densitometric analyses, n = 3, ****P < 0.0001 TFLLR 0.5 min and L-NAME + TFLLR 0.5 min versus Basal. (d) Total nitrate/nitrite production was measured using media from HCAECs pretreated with L-NAME (100 µmol/L) for 1 h prior to stimulation with TFLLR (10 µmol/L) for 0.5 min and 30 min, n = 3, TFLLR 0.5 min, no detection (nd) ****P < 0.0001 TFLLR 30 min and L-NAME + TFLLR 30 min versus basal
The PAR-1 specific activating peptide, TFLLR, produced similar results to thrombin showing a significant phosphorylation of eNOS-Ser-1177 at 30 s (P < 0.0001), which was not affected by the pretreatment with L-NAME (P < 0.0001), and TFLLR-induced eNOS-Ser-1177 phosphorylation was detectable below basal at the 30-min time point, and again was not inhibited by L-NAME in HCAECs (Figure 5(c)). In Figure 5(d), the nitrate/nitrite metabolites were undetectable when stimulated with TFLLR for 30 s in the absence and presence of L-NAME. However, a significant amount of nitrate/nitrite was measured in cells stimulated with TFLLR for 30 min (P < 0.0001). In the presence of L-NAME (Figure 5(d)), there was a significant decrease in the production of these NO metabolites (P < 0.0001). Overall, our data demonstrate that PAR-1 activation induces immediate phosphorylation of eNOS-Ser-1177 with eNOS being activated at a later time point in adult HCAECs, which, as stated earlier, may be due to input from the activation of eNOS-Thr-495.
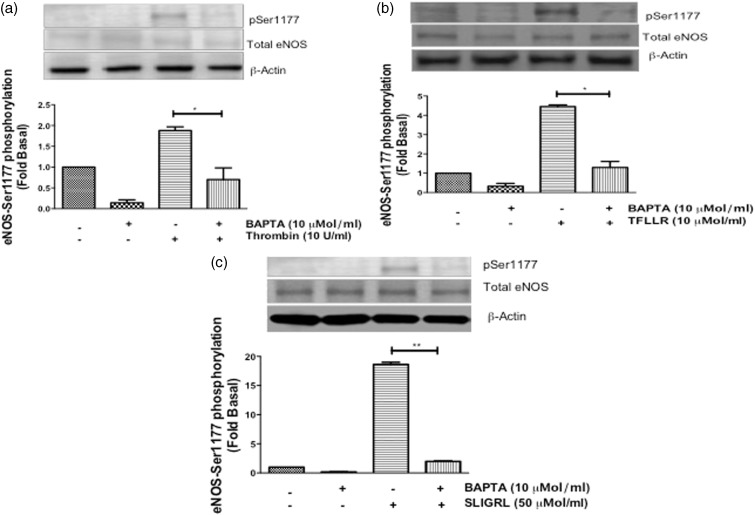
Effects of calcium inhibitor, BAPTA, on PAR-mediated eNOS-Ser-1177 phosphorylation in HCAECs
The Ca2+/calmodulin domain regulates all isoforms of NOS,16,18 specifically, thrombin has been shown to increase intracellular Ca2+ to induce eNOS-Ser-1177 phosphorylation and cGMP production.5,15,24,25 Therefore, we wanted to determine whether calcium was involved in the differential PAR-mediated phosphorylation of eNOS-Ser-1177 in HCAECs. Cells were pretreated with the selective intracellular Ca2+chelator, BAPTA (10 µmol/ml) for 30 min, and then stimulated with thrombin and TFLLR for 30 s, and SLIGRL for 2 min. Immunoblot analysis showed that BAPTA significantly inhibited thrombin- (P < 0.05) (Figure 6(a)), TFLLR- (P < 0.001) (Figure 6(b)) and SLIGRL-mediated (P < 0.001) (Figure 6(c)) eNOS-Ser-1177 phosphorylation. These data confirm that thrombin/PAR-mediated eNOS-Ser-1177 phosphorylation is contingent upon the mobilization of intracellular Ca2+ stores within HCAECs.
Figure 6.
Effects of calcium inhibitor, BAPTA, on PAR-mediated eNOS-Ser-1177 phosphorylation in HCAECs. HCAECs were pretreated with BAPTA-AM (10 µmol/L) for 30 min and then stimulated with (a) thrombin (10 U/ml) and (b) TFLLR (10 µmol/L) for 0.5 min, and (c) SLIGRL (50 µmol/L) for 2 min. Phosphorylation of eNOS-Ser1177 was determined using Western blot analysis with phosphospecific antibody. Representative immunoblots are shown with densitometric analyses. Mean +/− SEM of measurements in cultures of three different passages; *P < 0.05 Thrombin versus BAPTA + Thrombin and TFLLR versus BAPTA + TFLLR, **P < 0.001 SLIGRL versus BAPTA + SLIGRL, n = 3
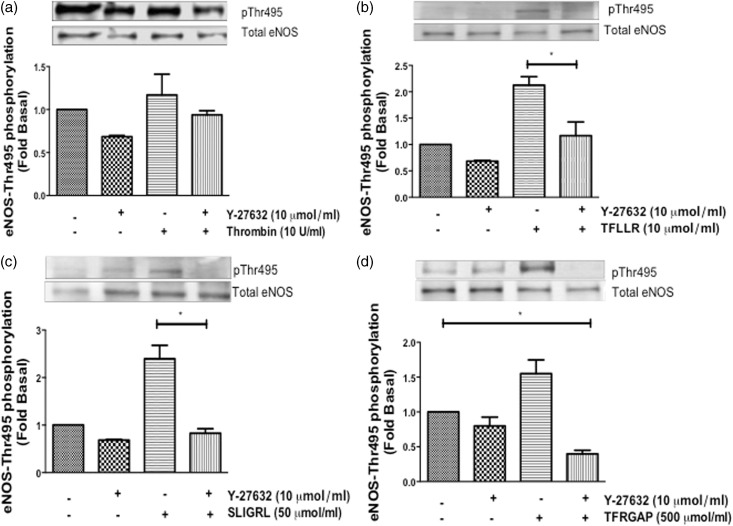
Effects of ROCK inhibitor, Y-27632, on PAR-mediated eNOS-Thr-495 phosphorylation in HCAECs
The involvement of the RhoA/ROCK pathway in endothelial dysfunction has been a major source of controversy.6,9,12,13 We used Y-27632 (Y-27), a ROCK inhibitor, to elucidate the mechanism by which activated PAR-1, -2, and -3 induces eNOS-Thr-495 phosphorylation. HCAECs were pretreated with Y-27 (10 µmol/L) for 90 min, and then stimulated with thrombin for 10 min, TFLLR for 2 min, and SLIGRL and TFRGAP for 10 min. Thrombin stimulation did not alter eNOS-Thr-495 phosphorylation regardless of the presence of Y-27 (Figure 7(a)). However, pretreatment with Y-27 significantly decreased selective TFLLR-induced (P < 0.05) (Figure 7(b)), as well as SLIGRL-induced (P < 0.05) (Figure 7(c)) and TFRGAP-induced (P < 0.05) (Figure 7(d)) eNOS-Thr-495 phosphorylation in HCAECs. These data confirm that eNOS-Thr-495 regulation is mediated via a ROCK-dependent pathway in HCAECs.
Figure 7.
Effects of ROCK inhibitor, Y-27632, on PAR-mediated eNOS-Thr-495 phosphorylation in HCAECs. HCAECs were pretreated with Y-27632 (10 µmol/L) for 90 min and then stimulated with (a) thrombin (10 U/ml) for 10 min, (b) TFLLR (10 µmol/L) for 2 min, (c) SLIGRL (50 µmol/L) for 10 min and (d) TRAGAP (500 µmol/L) for 10 min. Phosphorylation of eNOS-Thr-495 was determined using Western blot analysis with phosphospecific antibody. Representative immunoblots are shown with densitometric analyses. Mean +/− SEM of measurements in cultures of three different passages; *P < 0.05 TFLLR 2 min, SLIGRL 10 min, TFRGAP 10 min versus Basal, or Y-27 versus Y-27 + TFLLR 2 min, SLIGRL 10 min, or TFRGAP 10 min, n = 3
Discussion
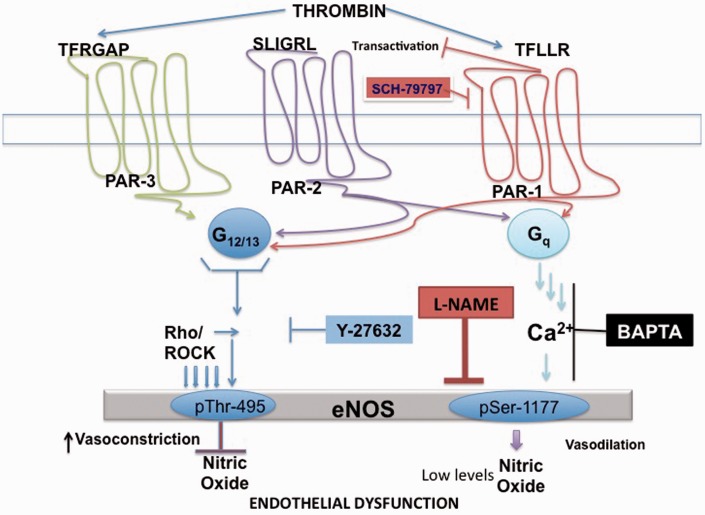
In this study, we have identified a variance in PAR coupling to signaling pathways that regulate eNOS phosphorylation and NO production in adult HCAECs. The principal findings are as follows: (1) a PAR-mediated biphasic signaling event that regulates eNOS through dual signaling pathways that results in simultaneous phosphorylation of both eNOS regulatory sites; (2) PAR-induced eNOS-Ser-1177 phosphorylation is Ca2+-dependent, which suggests a Gq-mediated mechanism,13,15,16 and results in a delayed increase in NO production, while PAR-induced eNOS-Thr-495 phosphorylation is ROCK-dependent, suggesting G12/13 coupling,12 which results in a decrease in NO production in HCAECs; and finally (3) we demonstrated for the first time that the elusive PAR-3 regulates eNOS-Thr-495 via a ROCK-dependent pathway. All of which strongly supports differential PAR signaling and altered eNOS regulation in the adult coronary arterial endothelium as demonstrated in Figure 8.
Figure 8.
Proposed signal transduction cascade of PAR-1, PAR-2 and PAR-3 in Adult HCAECs. In adult HCAECs, PAR-1 and PAR-2 couple to both Gq and G12/13 to regulate the signaling pathways that phosphorylate eNOS and cause NO production. A Ca2+-dependent-mediated mechanism, which is coupled to Gq, leads to the phosphorylation of eNOS-Ser-1177 and causes an increase in NO production. A ROCK-dependent pathway, which is coupled to G12/13, leads to eNOS-Thr-495 phosphorylation and a decrease in NO production. PAR-3 activation is coupled to G12/13 and the ROCK signaling pathway, which leads to a suppression of NO production via phosphorylation of eNOS-Thr-495. (A color version of this figure is available in the online journal.)
The attenuation of the endothelium-dependent dilator response as a result of aging, a major risk factor for the development of atherosclerosis, coronary artery disease and other cardiovascular events, is a consequence of the alteration in expression and/or activity of NO synthase.1,26 Our data show PAR-1 ligands, thrombin and TFLLR, induced phosphorylation of eNOS-Ser-1177 with a substantial delay in NO production in HCAECs, whereas our previous studies showed a PAR-2 ligand, SLIGRL, induced phosphorylation of eNOS-Ser-1177 followed by NO production in HUVEC,12 which supports the role of a given vascular environment in specific eNOS regulation. NO regulates normal blood pressure and its reduced bioavailability is considered a major cause of vascular disease27; however, high concentrations of NO can react negatively by combining with oxidants to produce superoxide (O2−) and peroxynitrite (ONOO−), which will lead to vascular inflammation and increased vascular permeability. Lowry et al.28 recently found that under inflammatory conditions eNOS-Ser-1177 is phosphorylated and can contribute to high NO output in the vasculature, which is normally attributed to inducible NOS (iNOS). Alternatively, at low levels, NO promotes barrier function, reduce cytokine recruitment, and enhance vascular stability.29 The use of L-NAME, an eNOS inhibitor, significantly blocked the thrombin/eNOS/NO signaling pathway confirming that eNOS is functional and responsible for NO production in HCAECs. In previous studies, we showed thrombin-induced cGMP production via eNOS activation was inhibited using an eNOS alternative L-NAME analog, NG-nitro-L-arginine methyl ester, which attenuated thrombin-induced cGMP production.15 The low and undetectable levels of NO shown in our studies suggest a hypertensive-like state and could be the result of cell aging, genetic modifications, and/or limited endothelial NOS regulation at the substrate and/or cofactor levels. Despite the two-fold increase in phosphorylation of eNOS-Ser-1177 shown with thrombin, TFLLR, and SLIGRL, NO production may be modulated by input from the phosphorylation of eNOS-Thr-495. The phosphorylation of this catalytic site was significantly elevated by TFLLR and SLIGRL, which resulted in a significant delay or complete inhibition in enhanced NO production, respectively. Our current methodology for NOS function is limited to NO byproduct, nitrates and nitrite, only as demonstrated in previous studies.12 Therefore, it is also possible that the reduced NO levels may be attributed to low bioavailability of the essential cofactor, tetrahydrobiopterin (BH4). It has been shown that trace amounts of BH4 will lead to eNOS uncoupling to produce reactive oxygen species (ROS), superoxide (O2−), which will exacerbate the endothelium in a negative feedback loop increasing ROS, vascular inflammation, and a severe vascular instability.30 It is known that eNOS uncoupling promotes “Nitroxidative Stress,” an event that enhances the expression of nitrotyrosine, a reactive nitrogen marker31; therefore, we measured the expression of this protein and found that it was not detected in any of our experimental conditions (data not shown). Techniques for direct measurement of other “nitro” species for eNOS function will be utilized in future studies. Low PAR-1 expression, thrombin concentration, or receptor recycling may also contribute to the difference in eNOS activity displayed in HCAECs.4,5,10 The attenuation of the endothelium-dependent vasodilation response as a consequence of the alteration in the expression and/or activity of eNOS is associated with aging vessels or disease development.26 In vivo studies support these findings showing that coronary artery occlusion triggers endothelial dysfunction and decreased eNOS-dependent vasoreactivity.32 A more recent study demonstrates impairment in endothelial-dependent vasodilation secondary to a loss of NO production from NOS with a shift of the enzyme to the production of O2−.33
It has been confirmed that thrombin, at low concentrations, induces eNOS-Thr-495 phosphorylation through its high-affinity receptor, PAR-1, whereas high thrombin concentrations induce transactivation of PAR-2 via PAR-1 tethered ligand leading to Gq coupling which mediates eNOS-Ser-1177 phosphorylation and immediate NO production.12,13 In HCAECs, activation of PAR-1 via thrombin has a minimal effect on eNOS-Thr-495 phosphorylation, which appears to be predominately regulated by PAR-2, and -3. Thrombin-mediated NO production is attenuated in the presence of the PAR-1 inhibitor, SCH, confirming the specificity of PAR-1 in the production of NO. Extended PAR-1 activation via thrombin in human pulmonary endothelial cells revealed a negative regulation of key players in the NO/cGMP pathway to promote pulmonary arterial hypertension.11 While HCAECs used in this study were classified as normal, they could have been derived from a stressed or a hypertensive endothelium state, demonstrating a PAR-mediated eNOS/NO dysfunction in primary arterial cells.17
Differential coupling of PARs in other cellular systems such as in HUVECs, which are embryonic and differentiate in nature shows PAR-2 activation with SLIGRL as a positive regulator of eNOS.12 Supporting this phenomenon, studies using BAECs revealed PAR-2 activation leads to increased phosphorylation at eNOS-Ser-1179 through a phospholipase C-dependent rapid and transient increase in intracellular Ca2+.15 In vivo studies have shown that systemic administration of a PAR-2 agonist yields a persistent hypotensive response due to arterial dilation with no change in heart rate.34 Similarly, short-term activation of PAR-2 has been described to have vasodilative properties in humans and rodents.35,36 This study challenged the established dogma of PAR-2 signaling, by demonstrating that PAR-2 activation leads to the phosphorylation of both regulatory sites in HCAECs with no increase in NO production, supporting PAR-2 could be a negative regulator of eNOS in certain human endothelial cells. Studies in smooth muscle cells attribute PAR-2 activation to increased MYPT phosphorylation and contractility.37 In addition, prolonged PAR-2 activation was characterized to cause endothelium dysfunction, independent of systemic pro-inflammation in vivo, supporting the diverse signaling potential of PAR-2 in the vasculature.35 This opposing role of PAR-1/-2 signaling demonstrated in this study provides a clue of disease initiation, an area to be explored in future studies.
Little is known about the function and signaling capacity of the second thrombin receptor, PAR-3. Initial studies in mouse platelets concluded that PAR-3 functions as a cofactor for PAR-4; mouse PAR-3 (mPAR-3) response was null to thrombin when over-expressed; however, inhibition of mPAR-3 and/or knockdown of the mPAR-3 gene inhibited mouse platelet activation at low levels of thrombin, suggesting mPAR-3 as a facilitator for mPAR-4 signaling.21 However, unlike PAR-4, PAR-3 is significantly expressed in HCAECs and other human vascular subtypes.5,10 Our data suggest that activated PAR-3 regulates the negative site of eNOS by increasing the phosphorylation of eNOS-Thr-495 in a time-dependent manner, via ROCK-mediated signaling; however, NO levels remain unchanged. The functional role for PAR-3 in the eNOS/NO pathway remains to be explored further. Non-classical activation of PAR-3 by Factor FXa, at Arg41 unlike Lys38 by thrombin, stabilizes tight junctions via Tie2 activation to enhance vascular stability in immortalized endothelial cells.38 Preliminary studies in our lab demonstrated that PAR-3 can signal independently of PAR-1, and act as the primary regulator of eNOS in a PAR-1 down-regulated HCAEC-PAR-1−/− cell line, which caused a transient phosphorylation of eNOS-Ser-1177 and a sustained phosphorylation of eNOS-Thr-495 (data not shown) in the presence of thrombin. Thrombin and TFRGAP were shown to signal via the ERK1/2 pathway to enhance the upregulation and release of IL-8 in PAR-3 over-expressed HEK-293 cells.6 On the contrary, McLaughlin et al.20 observed an increase in Gα13 coupling to PAR-1/PAR-3 heterodimers rather than PAR-1 homodimers, concluding that PAR-3 alloseterically regulates PAR-1 signaling to increase endothelial cell permeability, suggesting that PAR-3 has no independent signaling potential. More studies in primary human cells, healthy and diseased, will validate the pathophysiological relevance of PAR-3, making it a potential target for therapeutic manipulation.
Activation of PARs by thrombin prompts an array of intracellular signaling pathways, including PKC and Rho/ROCK.3,39 Studies using a specific Gq inhibitor, YM-254890, show that eNOS-Ser-1177 induced phosphorylation and NO production was blocked.13 In BAECs, thrombin-induced positive eNOS/NO modulation occurs via a Ca2+-dependent, PKCδ-sensitive, but PI3-K/AKT-independent pathway.15 In this study, we found that the phosphorylation of eNOS-Ser-1177, a site conserved in the Ca2+/calmodulin domain of eNOS, was blocked by BAPTA, an intracellular Ca2+ chelator, confirming the essential role of Ca2+ in eNOS-Ser-1177 phosphorylation and NO production.16,18,19 Alternatively, Rho has been established to block NO production in arteries40 and cease AKT signaling in endothelial cells to prevent eNOS-Ser-1177 phosphorylation.9 We were able to discern eNOS-Thr-495 regulation by inhibiting the downstream kinase of Rho, ROCK, with inhibitor Y-27632, which blocked PAR-1, PAR-2, and PAR-3 mediated eNOS-Thr-495 phosphorylation. Targeting RhoA by C3 exoenzyme, which inactivates Rho by ADP-ribosylation at the asparagine 41 in the effector region of the GTPase, recovered the down-regulation of eNOS expression induced by thrombin and suppressed TFLLR-induced eNOS-Thr-495 phosphorylation.12,39 All of which confirms that the constitutively active Rho/ROCK pathway enhances PAR-mediated negative eNOS function, likely suggesting G12/13 activity to suppress the Ca2+/calmodulin interaction of eNOS. Concluding that phosphorylated eNOS-Thr-495 down-regulates eNOS enzymatic activity; a PAR-2 and PAR-3 mediated response, indicating a reverse function in the adult human endothelium.
In conclusion, the findings in this study confound the established PAR-mediated signaling of vascular eNOS function and NO production. The well-known regulators of eNOS appear to exchange roles in human coronary endothelial cells, which could be the initiation of disease progression, establishing a novel PAR signal alteration. The expression ratio of PARs in primary HCAEC verses other subtypes/species vary and may also contribute to the reverse signaling demonstrated in this study. Endothelial derived NO and its derivatives play a critical role in regulating normal vascular function but can also be involved in exacerbating or eliminating cardiovascular diseases.29,41 In contrast to other studies, we found that PAR-1 and -2 can couple to both Ca2+ and ROCK signaling pathways to increase or suppress NO production, while also discerning the role of the elusive PAR-3, which negatively regulates eNOS via a ROCK-dependent mechanism. Complementary translational studies in distinct vascular beds need to be explored to fully ascertain PAR-mediated eNOS/NO signaling. Furthermore, understanding of PAR signaling in the human vasculature will be beneficial to look for new therapies to circumvent and treat endothelial dysfunction and other associated cardiovascular diseases.
Acknowledgements
The authors like to sincerely thank Jamill Jackson and Danielle Weekes for their technical assistances in this study. This work was supported in part by National Institutes of Health: Vanderbilt CTSA grant 1UL1RR024975-01 (L.Tillery), RCMI G12 MD007586 (Meharry Molecular Biology Core), SC3 SC3GM086336 (E.Motley), RISE R25-GM059994-15 (M. Lima) and T32-HL007737-21 (F. Villalta).
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; LCT and TAE conducted the experiments, EDM supplied critical reagents, LCT and EDM analyzed the results and LCT, EDM and SE wrote the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Brandes RP. Endothelial dysfunction and hypertension. Hypertension 2014; 64: 924–8. [DOI] [PubMed] [Google Scholar]
- 2.Kang KT. Endothelium-derived relaxing factors of small resistance arteries in hypertension. Toxicol Res 2014; 30: 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sidhu TS, French SL, Hamilton JR. Differential signaling by protease-activated receptors: implications for therapeutic targeting. Int J Mol Sci 2014; 15: 6169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000; 407: 258–64. [DOI] [PubMed] [Google Scholar]
- 5.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev 2001; 53: 245–82. [PubMed] [Google Scholar]
- 6.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev 2004; 84: 579–621. [DOI] [PubMed] [Google Scholar]
- 7.Russo A, Soh UJ, Trejo J. Proteases display biased agonism at protease-activated receptors: location matters!. Mol Interv 2009; 9: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 2005; 3: 1800–14. [DOI] [PubMed] [Google Scholar]
- 9.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol 2002; 22: 8467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soh UJ, Dores MR, Chen B, Trejo J. Signal transduction by protease-activated receptors. Br J Pharmacol 2010; 160: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickel KF, Laux V, Heumann R, von Degenfeld G. Thrombin has biphasic effects on the nitric oxide-cGMP pathway in endothelial cells and contributes to experimental pulmonary hypertension. PLoS One 2013; 8: e63504–e63504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watts VL, Motley ED. Role of protease-activated receptor-1 in endothelial nitric oxide synthase-Thr495 phosphorylation. Exp Biol Med (Maywood) 2009; 234: 132–9. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Motley ED, Eguchi K, Hinoki A, Shirai H, Watts V, Stemmle LN, Fields TA, Eguchi S. Distinct roles of protease-activated receptors in signal transduction regulation of endothelial nitric oxide synthase. Hypertension 2009; 53: 182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natarajan M, Konopinski R, Krishnan M, Roman L, Bera A, Hongying Z, Habib SL, Mohan S. Inhibitor-kappaB kinase attenuates Hsp90-dependent endothelial nitric oxide synthase function in vascular endothelial cells. Am J Physiol Cell Physiol 2015; 308: C673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motley ED, Eguchi K, Patterson MM, Palmer PD, Suzuki H, Eguchi S. Mechanism of endothelial nitric oxide synthase phosphorylation and activation by thrombin. Hypertension 2007; 49: 577–83. [DOI] [PubMed] [Google Scholar]
- 16.Hirano K, Nomoto N, Hirano M, Momota F, Hanada A, Kanaide H. Distinct Ca2+ requirement for NO production between proteinase-activated receptor 1 and 4 (PAR1 and PAR4) in vascular endothelial cells. J Pharmacol Exp Ther 2007; 322: 668–77. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Zhang J, Li B, Gao X, Liu Y, Mao W, Chen SL. Resveratrol ameliorates low shear stressinduced oxidative stress by suppressing ERK/eNOSThr495 in endothelial cells. Mol Med Rep 2014; 10: 1964–72. [DOI] [PubMed] [Google Scholar]
- 18.Wu PR, Chen BR, Hsieh CC, Lin WC, Wu KK, Hwu Y, Chen PF. The N-terminal portion of autoinhibitory element modulates human endothelial nitric-oxide synthase activity through coordinated controls of phosphorylation at Thr495 and Ser1177. Biosci Rep 2014; 34: e00129–e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen PF, Wu KK. Characterization of the roles of the 594-645 region in human endothelial nitric-oxide synthase in regulating calmodulin binding and electron transfer. J Biol Chem 2000; 275: 13155–63. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci U S A 2007; 104: 5662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature 2000; 404: 609–13. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara H, Connolly AJ, Zeng D, Kahn ML, Zheng YW, Timmons C, Tram T, Coughlin SR. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature 1997; 386: 502–6. [DOI] [PubMed] [Google Scholar]
- 23.Andersen H, Greenberg DL, Fujikawa K, Xu W, Chung DW, Davie EW. Protease-activated receptor 1 is the primary mediator of thrombin-stimulated platelet procoagulant activity. Proc Natl Acad Sci U S A 1999; 96: 11189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimura M, Oshima T, Matsuura H, Inoue T, Kambe M, Kajiyama G. Differential effects of extracellular Mg2+ on thrombin-induced and capacitative Ca2+ entry in human coronary arterial endothelial cells. Arterioscler Thromb Vasc Biol 1997; 17: 3356–61. [DOI] [PubMed] [Google Scholar]
- 25.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 2007; 42: 271–9. [DOI] [PubMed] [Google Scholar]
- 26.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res 2005; 66: 286–94. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee A, Black SM, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol 2008; 49: 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowry JL, Brovkovych V, Zhang Y, Skidgel RA. Endothelial nitric-oxide synthase activation generates an inducible nitric-oxide synthase-like output of nitric oxide in inflamed endothelium. J Biol Chem 2013; 288: 4174–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolluru GK, Siamwala JH, Chatterjee S. eNOS phosphorylation in health and disease. Biochimie 2010; 92: 1186–98. [DOI] [PubMed] [Google Scholar]
- 30.McNeill E, Channon KM. The role of tetrahydrobiopterin in inflammation and cardiovascular disease. Thromb Haemost 2012; 108: 832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radi R. Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res 2013; 46: 550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanBenthuysen KM, McMurtry IF, Horwitz LD. Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J Clin Invest 1987; 79: 265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci U S A 2007; 104: 15081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung WM, Andrade-Gordon P, Derian CK, Damiano BP. Receptor-activating peptides distinguish thrombin receptor (PAR-1) and protease activated receptor 2 (PAR-2) mediated hemodynamic responses in vivo. Can J Physiol Pharmacol 1998; 76: 16–25. [DOI] [PubMed] [Google Scholar]
- 35.Hughes KH, Wijekoon EP, Valcour JE, Chia EW, McGuire JJ. Effects of chronic in-vivo treatments with protease-activated receptor 2 agonist on endothelium function and blood pressures in mice. Can J Physiol Pharmacol 2013; 91: 295–305. [DOI] [PubMed] [Google Scholar]
- 36.McGuire JJ, Van Vliet BN, Gimenez J, King JC, Halfyard SJ. Persistence of PAR-2 vasodilation despite endothelial dysfunction in BPH/2 hypertensive mice. Pflugers Arch 2007; 454: 535–43. [DOI] [PubMed] [Google Scholar]
- 37.Sriwai W, Mahavadi S, Al-Shboul O, Grider JR, Murthy KS. Distinctive G protein-dependent signaling by protease-activated receptor 2 (PAR2) in smooth muscle: feedback inhibition of RhoA by cAMP-independent PKA. PLoS One 2013; 8: e66743–e66743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stavenuiter F, Mosnier LO. Noncanonical PAR3 activation by factor Xa identifies a novel pathway for Tie2 activation and stabilization of vascular integrity. Blood 2014; 124: 3480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eto M, Barandier C, Rathgeb L, Kozai T, Joch H, Yang Z, Luscher TF. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ Res 2001; 89: 583–90. [DOI] [PubMed] [Google Scholar]
- 40.Shiga N, Hirano K, Hirano M, Nishimura J, Nawata H, Kanaide H. Long-term inhibition of RhoA attenuates vascular contractility by enhancing endothelial NO production in an intact rabbit mesenteric artery. Circ Res 2005; 96: 1014–21. [DOI] [PubMed] [Google Scholar]
- 41.Searles CD. The nitric oxide pathway and oxidative stress in heart failure. Congest Heart Fail 2002; 8: 142–7, 155. [DOI] [PubMed] [Google Scholar]