Abstract
Sufentanil, a commonly used opioid analgesic, could mimic ischemia postconditioning to attenuate ischemia reperfusion injury, but this effect might be hindered in diabetic animals by inhibition of glycogen synthase kinase-3β phosphorylation. Also, diabetes can abrogate the cardioprotection of sevoflurane (an inhaled anesthetic) against ischemia reperfusion injury, and short-term insulin treatment does not restore protection by sevoflurane postconditioning. We hypothesized that long-term insulin treatment might restore the cardioprotective effect of sufentanil postconditioning in diabetic rats via phosphorylation of glycogen synthase kinase-3β. Streptozotocin (55 mg/kg)-induced diabetic rats received insulin (Novolin N, 6–8 u/d) for two days or two weeks, then were exposed to 30-min ischemia and 120-min reperfusion. Sufentanil postconditioning was performed 5 min before the onset of reperfusion. Controls included non-diabetic rats, sham surgery for ischemia/reperfusion, and sufentanil vehicle. Infarct size, cardiac troponin I, and phosphorylated glycogen synthase kinase-3β were examined. Sufentanil postconditioning reduced infarct size by 46% in non-diabetic rats (P < 0.001), but diabetes prevented this protective effect. Two-day insulin treatment was not effective, but two-week treatment reduced infarct size by 45% (P < 0.001), reduced cardiac troponin I by 33% (P < 0.001), and increased phosphorylated glycogen synthase kinase-3β levels (P < 0.001) in the diabetic sufentanil postconditioning group. In conclusion, sufentanil-induced cardioprotection was restored by long-term insulin treatment. The underlying mechanism may be increased phosphorylation of glycogen synthase kinase-3β.
Keywords: Anesthesia, anesthesiology, cardiovascular, diabetes, reperfusion, insulin
Introduction
Coronary heart disease is the leading cause of morbidity and mortality worldwide. Paradoxically, restoration of blood flow can have deleterious consequences by causing myocardial ischemia reperfusion injury (IRI).1 Current interventions that protect the heart from IRI involve activation of the reperfusion injury salvage kinase (RISK) pathway by ischemic pre-2 or postconditioning3 and pharmacological pre- or postconditioning. Postconditioning refers to a brief episode of ischemia during the onset of reperfusion after coronary occlusion. It has been well documented that ischemic postconditioning produces beneficial effects against IRI by reducing infarct size,3 accumulation of neutrophils,4 oxidative stress,5 and endothelial dysfunction,6 as well as inhibiting apoptotic-necrotic cell death. Phosphoinositide 3-kinase (PI3K)-protein kinase B (PKB)/Akt-glycogen synthase kinase-3β (GSK-3β) signaling plays an important role in ischemic postconditioning-induced protection. Phosphorylation of GSK-3β has emerged as an end effector step where multiple protective signaling pathways converge.7 Our previous studies indicate that sufentanil, a highly potent µ-opioid agonist, confers cardioprotection against IRI in non-diabetic rats by altering GSK-3β.8,9
As an independent risk factor for cardiovascular disease, diabetes mellitus makes the heart more susceptible to IRI,10–12 perhaps because prolonged hyperglycemia may cause formation of advanced glycation end products (AGEs) and oxidative stress. Animal models of myocardial infarction have demonstrated that diabetes mellitus can limit the efficacy of cardioprotective interventions.13,14 Reduced protection by ischemic pre- and postconditioning may be associated with RISK signaling pathways, such as the Akt, signal transducers and activators of transcription 3 (STAT3), and extracellular signal-regulated kinase (ERK).15 We showed that the protective effect of sufentanil is abrogated in diabetic rats with reduced GSK-3β phosphorylation.9 However, Drenger et al.16 demonstrated that the cardioprotective effect of sevoflurane postconditioning was not present in hearts from rats with type 1 diabetes mellitus and could not be restored by two days of insulin pretreatment. However, the influence of longer term insulin therapy on sufentanil postconditioning remains uncertain. The aims of the current study are to investigate whether sufentanil postconditioning could be maintained by short- or long-term insulin treatment in diabetic rats and to elucidate the role of GSK-3β in this process.
Materials and methods
Animal preparation
Diabetes was induced in male Sprague-Dawley rats (weight, 250–280 g, seven weeks of age) by a single i.p. injection of 55 mg/kg streptozotocin (STZ) (Sigma Aldrich, St Louis, MO) in 0.1 M citrate buffer (pH 4.0). Two days later, animals that maintained fasting blood glucose concentrations >300.6 mg/dL were considered to be hyperglycemic. Weight-matched control rats received injections of the citrate buffer. All animals were given free food and water access. Fasting blood glucose levels and body weight were measured at the onset of experiment. Heart weight was recorded after excision. Our experiments conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 85-23, revised 1996). Animal protocols were approved, and experiments were conducted in accordance with guidelines established by the Anhui Medical University Research Committee.
All animals were anesthetized with pentobarbital sodium (40 mg/kg, i.p.) and maintained by repeat doses of 25 mg/kg. After tracheal intubation, rats were artificially ventilated (TOPO Small Animal Ventilator, Kent Scientific, Torrington, CT, USA) in a pressure-controlled mode (peak inspiratory pressure: 15 cm H2O, respiratory rate: 75 breaths/min). Catheters were inserted into the left femoral vein for fluid or drug infusion and the right carotid artery for continuous monitoring of blood pressure and collection of blood samples. During the experiment, body temperature was maintained at 37 ± 1℃ with a heating pad.
Induction of IR
A left parasternal incision was made through the third and fourth ribs, and the pericardium was gently opened to expose the heart. The left anterior descending artery (LAD) was ligated using a 6-0 prolene suture. Rats were allowed to stabilize for 30 min before LAD occlusion. Myocardial ischemia was confirmed by cyanosis on the myocardial surface and electrocardiogram changes. The LAD coronary artery was occluded by a snare as previously described.9 The heart was subjected to 30-min of ischemia, followed by 120-min of reperfusion by releasing the snare. Heart rate (HR), mean arterial pressure (MAP), and rate pressure product (RPP) were recorded at baseline at 30 min of ischemia and at 120 min of reperfusion.
Experimental protocols
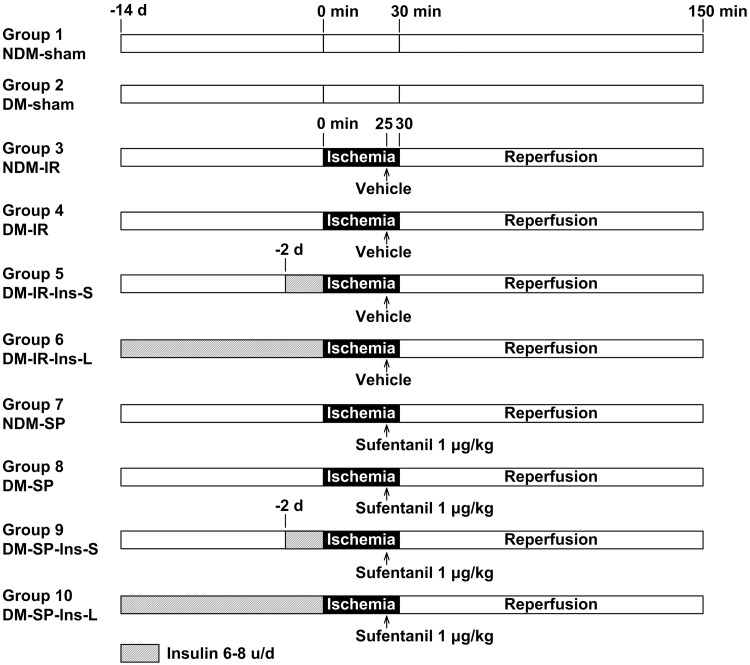
Rats were randomly assigned to one of 10 experimental groups with eight rats each, as shown in Figure 1: (1) non-diabetic sham (NDM-sham), (2) diabetic sham (DM-sham), (3) non-diabetic ischemia/reperfusion (NDM-IR), (4) diabetic ischemia/reperfusion (DM-IR), (5) diabetic ischemia/reperfusion with short-term insulin treatment (DM-IR-Ins-S), (6) diabetic ischemia/reperfusion with long-term insulin treatment (DM-IR-Ins-L), (7) non-diabetic ischemia/reperfusion with sufentanil postconditioning (NDM-SP), (8) diabetic ischemia/reperfusion with sufentanil postconditioning (DM-SP), (9) diabetic ischemia/reperfusion with sufentanil postconditioning and short-term insulin pretreatment (DM-SP-Ins-S), and (10) diabetic ischemia/reperfusion with sufentanil postconditioning and long-term insulin treatment (DM-SP-Ins-L). Short- and long-term insulin treatment groups received s.c. insulin (Novolin N [Nova Nordisk, Bagsvaerd, Denmark]) at 6–8 units per day for 2 or 14 days, respectively. On the experimental day, all groups were subjected to 30 min of ischemia and 120 min of reperfusion, except the two sham groups. Sufentanil postconditioning groups received sufentanil (1 µg/kg, i.v.) 5 min before the onset of reperfusion. Sham and control groups were given vehicle only.
Figure 1.
Experimental groups with their respective protocols. n = 8 per group.
NDM: non-diabetic rats; DM: diabetic rats; IR: ischemia/reperfusion; SP: sufentanil postconditioning; Ins-S: two-day short-term insulin treatment; Ins-L: two-week long-term insulin treatment.
The experiment was repeated in a separate cohort of animals. Hearts from the first cohort were used to calculate infarct size relative to the area at risk (AAR), using double labeling with 2,3,5,-triphenyltetrazolium chloride (Sigma Chemical, St. Louis, MO) and Evans blue dye (Sigma Chemical, St. Louis, MO). Hearts from the second cohort were stored in liquid nitrogen until being prepared for Western blotting.
Assessment of biochemical parameters
At the end of the 120-min reperfusion, blood was collected from the carotid artery immediately before the heart was excised. Cardiac troponin I (cTnI) levels were measured using ELISA kits (R&D Systems, Minneapolis, MN, USA).
Determination of infarct size
At the end of the 120-min reperfusion, the heart was excised and weighed. The left coronary artery was reoccluded, and the aortic root was reperfused with 0.4 mL of 0.5% Evans blue dye, which stained the normal tissue. The heart was then frozen at −20℃ before being sliced into five transverse sections (thickness, 1 mm). The slices were incubated (37℃) for 15 min in buffered 1% 2,3,5,-triphenyltetrazolium chloride, adjusted to pH 7.4, and then immersed for 15 min in 4% formaldehyde. This procedure stained the viable AAR red and normal tissue blue, leaving the infarct area unstained. The AAR and infarct area were quantified using planimetry Image J software (NIH, Bethesda, MD, USA).
Western blot analysis
For analysis of GSK-3β phosphorylation, hearts were collected from all 10 groups (n = 4 per group). All animals were subjected to 30 min of left coronary artery occlusion and reperfusion. At 5 min after reperfusion, hearts were excised, and the left ventricles were immediately frozen in liquid nitrogen and stored at −80℃. Phosphorylated and total GSK-3β levels were analyzed as previously described15 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using antibodies obtained from Abcam (Cambridge, UK) and diluted 1:1000. Phosphorylated GSK-3β levels were normalized against total GSK-3β. Equal protein loading was confirmed by glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative densitometry was determined using Image J software.
Statistical analysis
Data are reported as mean ± SD. A power analysis showed that eight animals per group was sufficient to detect a 40–50% reduction in IS/AAR with a power of 80% and a significance level of 0.05. Group differences among non-diabetic, diabetic, and diabetic + insulin animals for body weight, heart weight, and blood glucose levels were analyzed by one-way analysis of variance (ANOVA). Infarct size and Western blot results were analyzed by one-way ANOVA followed by Bonferroni post-hoc test for multiple comparisons. Hemodynamic data were analyzed by two-way ANOVA with repeated measurements. Statistical significance was defined as P < 0.05.
Results
Animal data
A total of 135 rats were initially included in the experiment. Six rats died after STZ-injection, and nine died during the process of ischemia or reperfusion. Of the remaining 120 rats, 80 were used to determine infarct size using triphenyltetrazolium chloride and Evans blue dye dual staining, and 40 were used for Western blot analysis of rat heart tissue sections.
As shown in Table 1, one week after receiving STZ (55 mg/kg), diabetic rats showed a 12% decrease in body weight and marked increases in fasting plasma glucose (426.6 to 541.8 mg/dL), compared with non-diabetic rats (P < 0.05). Both short-and-long-term insulin treatment lowered glucose levels. After two weeks of insulin treatment, glucose levels were equal to those in the non-diabetic group, and body weight increased by 12%(P < 0.05). In the two days insulin treatment group, glucose levels were higher and body weight was lower than the non-diabetic group (P < 0.05).
Table 1.
Characteristics of non-diabetic (NDM) and diabetic (DM) rats
| Bodyweight (g) | Heart weight (g) | Heart weight/ Body weight × 100 (%) | Blood glucose level (mg/dL) | |
|---|---|---|---|---|
| NDM (n = 36) | 314 ± 18 | 1.032 ± 0.076 | 0.329 ± 0.020 | 127.8 ± 16.2 |
| DM without insulin treatment (n = 36) | 279 ± 22a | 0.967 ± 0.062 | 0.349 ± 0.024 | 484.2 ± 57.6a |
| DM with two-day insulin treatment (n = 24) | 294 ± 11a | 1.010 ± 0.076 | 0.344 ± 0.024 | 262.8 ± 41.4a,b |
| DM with two-week insulin treatment (n = 24) | 353 ± 15a,b,c | 1.206 ± 0.071a,b,c | 0.342 ± 0.024 | 127.8 ± 25.2b,c |
Data were analyzed between groups using one-way ANOVA. Bodyweight and blood glucose level were measured at the day of experiment and heart weight were measured immediately after excision. Data are presented as mean ± SD.
NDM: non-diabetic rats; DM: diabetic rats; Ins-S: two-day short-term insulin treatment; Ins-L: two-week long-term insulin treatment.
P < 0.05 compared with the NDM group; bP < 0.05 compared with the DM without insulin treatment; cP < 0.05 compared with the DM with two-day insulin treatment group.
Hemodynamic parameters
HR, MAP, and RPP for all groups are displayed in Table 2. No significant group differences were seen at baseline. All groups showed decreased HR, MAP, and RPP after induction of ischemia/reperfusion, except DM sham and NDM sham groups. Compared with sham groups, HR, MAP, and RPP decreased significantly after 120 min of reperfusion in all ischemia/reperfusion groups (P < 0.05). Other values showed no other statistically significant inter- or intragroup comparisons.
Table 2.
Hemodynamics at baseline at 30 min of ischemia and at 120 min of reperfusion in all groups
| Group | HR |
MAP (mmHg) |
RPP (mmHg/min/1000) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 30 min- ischemia | 120 min- reperfusion | Baseline | 30 min- ischemia | 120 min- reperfusion | Baseline | 30 min- ischemia | 120 min- reperfusion | |
| NDM-sham | 384 ± 7 | 381 ± 11 | 373 ± 9a | 101 ± 4 | 97 ± 3a | 90 ± 4a | 38 ± 1 | 37 ± 2a | 36 ± 2a |
| DM-sham | 389 ± 13 | 382 ± 10b | 372 ± 10b | 97 ± 3 | 94 ± 4b | 87 ± 4b | 38 ± 2 | 37 ± 2b | 35 ± 2b |
| NDM-IR | 384 ± 13 | 367 ± 20 | 332 ± 17c,d | 95 ± 4 | 84 ± 5 | 66 ± 5c,d | 36 ± 3 | 31 ± 3c | 22 ± 2c,d |
| DM-IR | 365 ± 11 | 345 ± 13c | 307 ± 14c,d | 96 ± 4 | 76 ± 3c | 62 ± 2c,d | 35 ± 1 | 26 ± 2c | 19 ± 1c,d |
| DM-IR-Ins-S | 363 ± 14 | 345 ± 17 | 307 ± 16c,d | 98 ± 5 | 78 ± 7c | 69 ± 5c,d | 36 ± 2 | 27 ± 3c | 21 ± 2c,d |
| DM-IR-Ins-L | 369 ± 20 | 340 ± 15c | 305 ± 19c,d | 100 ± 5 | 81 ± 9c | 67 ± 5c,d | 37 ± 4 | 27 ± 4c | 21 ± 3c,d |
| NDM-SP | 381 ± 26 | 351 ± 24 | 323 ± 25c | 95 ± 6 | 81 ± 7c | 73 ± 7c | 36 ± 3 | 28 ± 2c | 24 ± 3c,d |
| DM-SP | 378 ± 13 | 358 ± 15 | 329 ± 22c,d | 102 ± 5 | 82 ± 5c | 67 ± 4c,d | 38 ± 1 | 29 ± 2c | 22 ± 2c,d |
| DM-SP-Ins-S | 371 ± 12 | 359 ± 21 | 322 ± 16c,d | 100 ± 7 | 83 ± 4c | 71 ± 5c,d | 37 ± 4 | 30 ± 2c | 23 ± 2c,d |
| DM-SP-Ins-L | 367 ± 22 | 337 ± 17c | 304 ± 8c,d | 96 ± 7 | 75 ± 9c | 64 ± 7c | 35 ± 5 | 25 ± 3c | 19 ± 2c,d |
Data were analyzed in a two-way ANOVA with repeated measurements. All values are expressed as mean ± SD., n = 8 per group.
HR: heart rate; MAP: mean artery pressure (mmHg); RPP: rate pressure product (mmHg/min/1000); NDM: non-diabetic rats; DM: diabetic rats; IR: ischemia/reperfusion; SP: sufentanil postconditioning; Ins-S: two-day short-term insulin treatment; Ins-L: two-week long-term insulin treatment.
P < 0.05 vs. NDM-IR group; bP < 0.05 vs. DM-IR group; cP < 0.05 vs. baseline; dP < 0.05 vs. 30-min ischemia.
Myocardial infarct size and cTnI
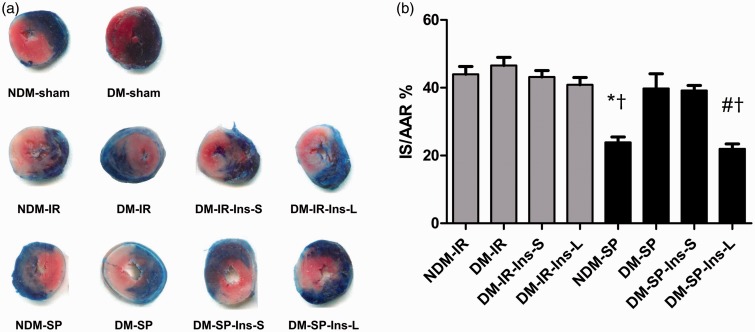
As shown in Table 3 and Figure 2, cTnI concentration, a specific indicator of myocardial injury, increased after ischemia/reperfusion compared to sham groups. In non-diabetic rats, sufentanil reduced infarct size (expressed as a percentage of the AAR) and plasma cTnI concentrations by 46% (NDM-SP vs. NDM-IR: 0.239 ± 0.036 vs. 0.440 ± 0.057, P < 0.001) and 30% (NDM-SP vs. NDM-IR: 3.34 ± 0.21 vs. 4.77 ± 0.14 µg/L, P < 0.001), respectively. However, sufentanil failed to attenuate infarct size and plasma cTnI elevation in diabetic rats. In diabetic rats treated with sufentanil, long-term insulin treatment reduced infarct size and plasma cTnI by 45% (DM-SP-Ins-L vs. DM-SP: 0.220 ± 0.039 vs. 0.398 ± 0.087, P < 0.001) and 33% (DM-SP-Ins-L vs. DM-SP: 3.59 ± 0.27 vs. 5.29 ± 0.18 µg/L, P < 0.001). Short-term insulin treatment did not reduce infarct size (P > 0.05). Neither short- nor long-term insulin treatment affected infarct size in diabetic rats that exposed to IRI but did not receive sufentanil (P > 0.05 both).
Table 3.
The infarct size and plasma concentration of cardiac troponin I (cTnI)
| Group | LV + RV (cm3) | IS (cm3) | AAR (cm3) | IS/AAR | cTnI (µg/L) |
|---|---|---|---|---|---|
| NDM-sham | 1.012 ± 0.056 | _ | 0.394 ± 0.032 | _ | 2.44 ± 0.28a |
| DM-sham | 1.019 ± 0.085 | _ | 0.396 ± 0.074 | _ | 2.88 ± 0.22b |
| NDM-IR | 0.995 ± 0.127 | 0.166 ± 0.013 | 0.383 ± 0.064 | 0.440 ± 0.057 | 4.77 ± 0.14 |
| DM-IR | 1.055 ± 0.069 | 0.167 ± 0.013 | 0.377 ± 0.055 | 0.447 ± 0.035 | 5.13 ± 0.11 |
| DM-IR-Ins-S | 1.068 ± 0.030 | 0.153 ± 0.005 | 0.358 ± 0.043 | 0.432 ± 0.042 | 4.91 ± 0.19 |
| DM-IR-Ins-L | 0.940 ± 0.086 | 0.151 ± 0.020 | 0.354 ± 0.042 | 0.431 ± 0.070 | 4.92 ± 0.18 |
| NDM-SP | 1.004 ± 0.055 | 0.098 ± 0.017a,c | 0.410 ± 0.050 | 0.239 ± 0.036a,c | 3.34 ± 0.21a,c |
| DM-SP | 0.988 ± 0.048 | 0.167 ± 0.019 | 0.427 ± 0.046 | 0.398 ± 0.087 | 5.29 ± 0.18 |
| DM-SP-Ins-S | 1.004 ± 0.115 | 0.141 ± 0.023 | 0.363 ± 0.070 | 0.392 ± 0.034 | 4.84 ± 0.13 |
| DM-SP-Ins-L | 0.985 ± 0.089 | 0.090 ± 0.012b,c | 0.419 ± 0.076 | 0.220 ± 0.039b,c | 3.59 ± 0.27b,c |
Data were analyzed between groups using one-way ANOVA. All values are expressed as mean ± SD., n = 8 per group.
LV + RV: sum of left and right ventricular area; IS: infarct size; AAR: area at risk; IS/AAR: infarct size as a percentage of AAR; NDM: non-diabetic rats; DM: diabetic rats; IR: ischemia/reperfusion; SP: sufentanil postconditioning; Ins-S: two-day short-term insulin treatment; Ins-L: two-week long-term insulin treatment; –: no detection.
P < 0.05 vs. NDM-IR group; bP < 0.05 vs. DM-IR group; cP < 0.05 vs. DM-SP group.
Figure 2.
Representative pictures of infarct size. (a) Transverse heart slices from rats by triphenyltetrazolium chloride staining; (b) bar graph shows mean ± SD of area at infarct as percentage of the area at risk by triphenyltetrazolium chloride staining, n = 8 per group.
NDM: non-diabetic rats; DM: diabetic rats; IR: ischemia/reperfusion; SP: sufentanil postconditioning; Ins-S: two-day short-term insulin treatment; Ins-L: two-week long-term insulin treatment.
*P < 0.05 vs. NDM-IR group; #P < 0.05 vs. DM-IR group; †P < 0.05 vs. DM-SP group. (A color version of this figure is available in the online journal.)
GSK-3β Western blot analysis
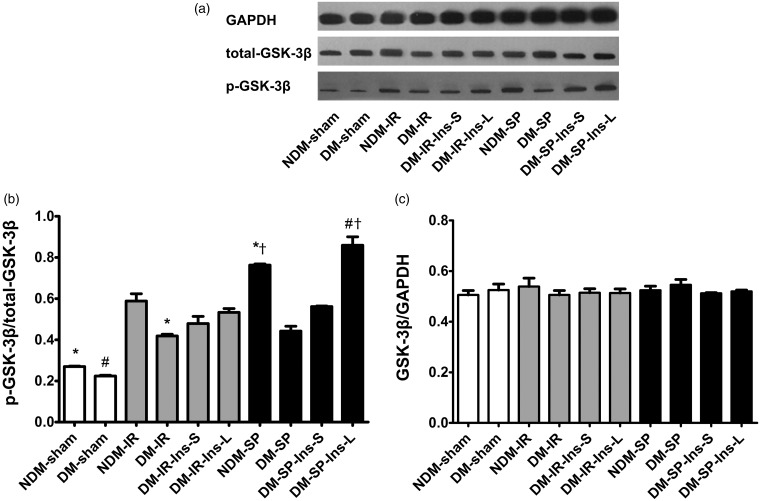
The phosphorylation GSK-3β at 5 min after reperfusion is illustrated by a representative Western blot and bar graph in Figure 3. Total GSK-3β was similar across groups. Therefore, all GSK-3β levels were normalized as a ratio to total GSK-3β. Sufentanil increased p-GSK-3β in non-diabetic rats (NDM-SP vs. NDM-IR: 0.763 ± 0.008 vs. 0.588 ± 0.050, P = 0.013) but not in diabetic rats. Long-term insulin treatment markedly increased p-GSK-3β levels (DM-SP-Ins-L vs. DM-SP: 0.860 ± 0.058 vs. 0.442 ± 0.033, P < 0.001), which was in accordance with infarct size.
Figure 3.
Expression of GSK-3β and p-GSK-3β in rat myocardium at the end of 120-min reperfusion. (a) Cardiac tissues were collected for detecting GSK-3β and p-GSK-3β expressions by Western blotting; (b) bar graph represents averaged total-GSK-3β expressed as percentage of GAPDH and as mean ± SD., n = 4 per group; (c) bar graph represents averaged p-GSK-3β as percentage of total-GSK-3β and expressed as mean ± SD., n = 4 per group.
NDM: non-diabetic rats; DM: diabetic rats; IR: ischemia/reperfusion; SP: sufentanil postconditioning; Ins-S: two-day short-term insulin treatment; Ins-L: two-week long-term insulin treatment.
*P < 0.05 vs. NDM-IR group; #P < 0.05 vs. DM-IR group; †P < 0.05 vs. DM-SP group
Discussion
We found that long- but not short-term insulin pretreatment, combined with sufentanil postconditioning, was cardioprotective in diabetic rats, as assessed by reduced infarct size and a comparable reduction in plasma cTnI concentration. These findings suggest three important conclusions. First, sufentanil postconditioning is cardioprotective in healthy control animals and the protection is hindered by diabetes, which is consistent with our previous experiment.9 Second, short-term insulin treatment (two days) is not sufficient to restore the cardioprotective effect of sufentanil, but long-term insulin treatment (two weeks) is sufficient. Third, the beneficial effects of long-term insulin treatment are associated with increased GSK-3β phosphorylation at Ser9 during sufentanil postconditioning.
The dose of sufentanil administered (1 µg/kg) was based on our previous study demonstrating a cardioprotective effect in non-diabetic but not diabetic rats.8,9 Neutral protamine Hagedorn (NPH) insulin is widely used in diabetic patients. We utilized Novolin N (6–8 u/d) in this study based on the results of Fang et al.17 and our pilot experiment. After treatment with STZ, rats showed severe hyperglycemia, polyuria (frequent urination), polydipsia (increased thirst), polyphagia (increased hunger), and weight loss. However, these symptoms reversed after treatment with insulin for two weeks. Weight gain was observed after long-term insulin treatment, possibly due to increased calorie intake.18
Hyperglycemia causes a loss of cardioprotective mechanisms by increasing inflammation, oxidative stress, and mitochondrial dysfunction. Many studies have shown that hyperglycemia abolishes cardioprotection by ischemic and pharmacological postconditioning.19 It is unclear whether this protective effect can be restored by normalization of glucose levels. The response of injured myocardium to normoglycemia and hyperglycemia may vary with age, species, and models. Clinical trials have demonstrated good outcomes with intensive glucose control by insulin.20 Przyklenk et al.21 also found in the type 1 diabetes mellitus mice that protective effects of ischemic postconditioning were reversed by the restoration of normoglycemia. However, in a study by Drenger et al., diabetic inhibition of protection by ischemic postconditioning was not relieved by insulin-induced normoglycemia.16 Aguilar et al.22 found that glucose lowering drug metformin might have a cardioprotective effect independent of its glucose lowering effect. Indeed, in our study, insulin lowered plasma glucose levels in diabetic rats. However, beneficial effects of insulin itself on infarct size and p-GSK-3β levels were not apparent in the absence of sufentanil. Hence, we speculate that cardioprotection in diabetic rats treated with long-term insulin and sufentanil cannot be attributed to normoglycemia. The “metabolic cocktail” comprising glucose–insulin–potassium (GIK) as a therapy against IR injury has been long accepted.23 Insulin is the key component of GIK in cardioprotection, and it protects ischemic heart against apoptotic cell death and improves myocardial survival via activating cell-survival signaling.23,24 Animal models showed a cardioprotective effect of receiving insulin at reperfusion, which was comparable to that of GIK.25 Clearly, insulin itself has cardioprotective effect.26 It directly increases cardiac contractility, reduces oxidation apoptosis, and inflammation responses via RISK pathways, and the downstream signaling molecules involve PI3K, PKB or (Akt), and GSK-3β.23 Furthermore, insulin counteracts glucose toxicity and thus indirectly tempers the devastating effects of hyperglycemia. To test the role of insulin, we compared diabetic IR rats with or without insulin treatment and found no difference on infarct size or p-GSK-3β. Thus, we speculate that insulin alone, at least in our study, is not cardioprotective. Taken together, we conclude that cardioprotection is mainly due to restored a sufentanil postconditioning effect, which is made possible by long-term insulin pretreatment.
Studies have shown that the diabetic heart is more susceptible to IR injury, but more refractory to cardioprotective interventions.27 Ichinomiya et al.28 found that low-dose fasudil could not protect the heart from IR injury in hyperglycemic rats, but higher doses were effective. Drenger et al.16 showed that the protective effect of ischemic preconditioning and sevoflurane postconditioning were not restored by two-day insulin treatment in diabetic rats. We showed that two-day insulin treatment was not effective to restore sufentanil postconditioning, but two-week treatment was sufficient. Thus, interventions to protect the diabetic heart should carefully examine dose and duration of treatment.
We found that sufentanil increased p-GSK-3β levels in non-diabetic rats, which is in agreement with other studies that have implicated inactivation of GSK-3β by phosphorylation at Ser9 as a common mechanism of cardioprotective interventions.29,30 It has been reported that GSK-3β activity is a determinant of the threshold for the mitochondrial permeability transition pore (mPTP) opening in cardiomyocytes.30,31 The mechanisms might be as follows29: GSK-3β induces hexokinase II release, which stabilizes mPTP; the binding of p-GSK-3β to adenine nucleotide translocase (ANT) suppresses interaction of ANT with CypD, a trigger of mPTP opening; p53-mediated regulation of mPTP might be suppressed by inhibition of GSK-3β activity; inhibition of GSK-3β suppresses ATP hydrolysis, inhibiting mPTP opening. However, in diabetic models, GSK-3β is more often activated due to impairment of upstream pathways, so the protective interventions are not effective.15,32 Methods aimed at inactivating the GSK-3β pathway, such as GSK-3β inhibitors or indirect activation of PI3K/Akt, may preserve preconditioning- or postconditioning-induced cardioprotection.33 SB216763, a selective GSK-3β inhibitor, was found to increase p-GSK-3β levels and reduce infarct size and cTnI levels when administered 5 min before reperfusion in diabetic models.9,15 In our study, long-term but not short-term insulin treatment supported restored cardioprotection. We believe that long-term insulin treatment inactivated GSK-3β by phosphorylation at Ser9, “desensitized” the mitochondria, and thus elevated the threshold for mPTP opening, while short-term insulin administration had no effect on that threshold.
Some limitations of this study should be noted. First, we only examined phosphorylation of GSK-3β, but did not use an inhibitor to suppress GSK-3β or PI3K. Thus, upstream and downstream effects of p-GSK-3β are unknown. Second, the two courses of insulin treatment in our study, two days and two weeks, both constitute acute insulin therapy, and a longer treatment course should be performed to investigate the effects of chronic insulin on IR injury. Future studies should aim to elucidate the mechanisms of how diabetes interacts with cardioprotective pathways and translate this information into clinical interventions.
In conclusion, the current study showed that in diabetic rats long-term insulin pretreatment restored the full cardioprotective effect of sufentanil postconditioning observed in non-diabetic rats. Long-term insulin treatment was associated with phosphorylation of GSK-3β, supporting this pathway as a target for therapeutic intervention.
Acknowledgements
This study was funded by the National of Nature Science Foundation of China (81341014) and the Youth Culture Program of First Affiliated Hospital of Anhui Medical University of China (3101005002352).
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; YZ and LZ contributed equally to this work.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007; 357: 1121–35. [DOI] [PubMed] [Google Scholar]
- 2.Otani H. Ischemic preconditioning: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2008; 10: 207–47. [DOI] [PubMed] [Google Scholar]
- 3.Wu QL, Shen T, Shao LL, Ma H, Wang JK. Ischemic postconditioning mediates cardioprotection via PI3K/GSK-3beta/beta-catenin signaling pathway in ischemic rat myocardium. Shock 2012; 38: 165–9. [DOI] [PubMed] [Google Scholar]
- 4.Mykytenko J, Kerendi F, Reeves JG, Kin H, Zatta AJ, Jiang R, Guyton RA, Vinten-Johansen J, Zhao ZQ. Long-term inhibition of myocardial infarction by postconditioning during reperfusion. Basic Res Cardiol 2007; 102: 90–100. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy D. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia–reperfusion injury. Cardiovasc Res 2003; 60: 617–25. [DOI] [PubMed] [Google Scholar]
- 6.Loukogeorgakis SP, Panagiotidou AT, Yellon DM, Deanfield JE, MacAllister RJ. Postconditioning protects against endothelial ischemia-reperfusion injury in the human forearm. Circulation 2006; 113: 1015–9. [DOI] [PubMed] [Google Scholar]
- 7.Rahman S, Li J, Bopassa JC, Umar S, Iorga A, Partownavid P, Eghbali M. Phosphorylation of GSK-3beta mediates intralipid-induced cardioprotection against ischemia/reperfusion injury. Anesthesiology 2011; 115: 242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Gu EW, Zhu Y, Zhang L, Liu XQ, Fang WP. Sufentanil limits the myocardial infarct size by preservation of the phosphorylated connexin 43. Int Immunopharmacol 2012; 13: 341–6. [DOI] [PubMed] [Google Scholar]
- 9.Chen QL, Gu EW, Zhang L, Cao YY, Zhu Y, Fang WP. Diabetes mellitus abrogates the cardioprotection of sufentanil against ischaemia/reperfusion injury by altering glycogen synthase kinase-3beta. Acta Anaesthesiol Scand 2013; 57: 236–42. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Liu Z, Wang J, Wong GT, Cheung CW, Zhang L, Chen C, Xia Z, Irwin MG. Susceptibility to myocardial ischemia reperfusion injury at early stage of type 1 diabetes in rats. Cardiovasc Diabetol 2013; 12: 133–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przyklenk K, Maynard M, Greiner DL, Whittaker P. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal 2011; 14: 781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engbersen R, Riksen NP, Mol MJ, Bravenboer B, Boerman OC, Meijer P, Oyen WJ, Tack C, Rongen GA, Smits P. Improved resistance to ischemia and reperfusion, but impaired protection by ischemic preconditioning in patients with type 1 diabetes mellitus: a pilot study. Cardiovasc Diabetol 2012; 11: 124–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HS, Cho JE, Hwang KC, Shim YH, Lee JH, Kwak YL. Diabetes mellitus mitigates cardioprotective effects of remifentanil preconditioning in ischemia-reperfused rat heart in association with anti-apoptotic pathways of survival. Eur J Pharmacol 2010; 628: 132–9. [DOI] [PubMed] [Google Scholar]
- 14.van den Brom CE, Bulte CS, Loer SA, Bouwman RA, Boer C. Diabetes, perioperative ischaemia and volatile anaesthetics: consequences of derangements in myocardial substrate metabolism. Cardiovasc Diabetol 2013; 12: 42–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross ER, Hsu AK, Gross GJ. Diabetes abolishes morphine-induced cardioprotection via multiple pathways upstream of glycogen synthase kinase-3beta. Diabetes 2007; 56: 127–36. [DOI] [PubMed] [Google Scholar]
- 16.Drenger B, Ostrovsky IA, Barak M, Nechemia-Arbely Y, Ziv E, Axelrod JH. Diabetes blockade of sevoflurane postconditioning is not restored by insulin in the rat heart: phosphorylated signal transducer and activator of transcription 3- and phosphatidylinositol 3-kinase-mediated inhibition. Anesthesiology 2011; 114: 1364–72. [DOI] [PubMed] [Google Scholar]
- 17.Fang D, Guan H, Liu J, Wei G, Ke W, Yao B, Xiao H, Li Y. Early intensive insulin therapy attenuates the p38 pathway in the renal cortex and indices of nephropathy in diabetic rats. Endocr J 2012; 59: 81–90. [DOI] [PubMed] [Google Scholar]
- 18.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes-causes, effects and coping strategies. Diabetes Obes Metab 200; 9: 799–812. [DOI] [PubMed] [Google Scholar]
- 19.Huhn R, Heinen A, Weber NC, Hollmann MW, Schlack W, Preckel B. Hyperglycaemia blocks sevoflurane-induced postconditioning in the rat heart in vivo: cardioprotection can be restored by blocking the mitochondrial permeability transition pore. Br J Anaesth 2008; 100: 465–71. [DOI] [PubMed] [Google Scholar]
- 20.Gerstein HC, Miller ME, Ismail-Beigi F, Largay J, McDonald C, Lochnan HA, Booth GL. Effects of intensive glycaemic control on ischaemic heart disease: analysis of data from the randomised, controlled ACCORD trial. Lancet 2014; 384: 1936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przyklenk K, Maynard M, Greiner DL, Whittaker P. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal 2011; 14: 781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ Heart Fail 2011; 4: 53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res 2001; 89: 1191–8. [DOI] [PubMed] [Google Scholar]
- 24.Gao F. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation 2002; 105: 1497–502. [DOI] [PubMed] [Google Scholar]
- 25.Zhang HF, Fan Q, Qian XX, Lopez BL, Christopher TA, Ma XL, Gao F. Role of insulin in the anti-apoptotic effect of glucose-insulin-potassium in rabbits with acute myocardial ischemia and reperfusion. Apoptosis 2004; 9: 777–83. [DOI] [PubMed] [Google Scholar]
- 26.Ng KW, Allen ML, Desai A, Macrae D, Pathan N. Cardioprotective effects of insulin: how intensive insulin therapy may benefit cardiac surgery patients. Circulation 2012; 125: 721–8. [DOI] [PubMed] [Google Scholar]
- 27.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 2007; 59: 418–58. [DOI] [PubMed] [Google Scholar]
- 28.Ichinomiya T, Cho S, Higashijima U, Matsumoto S, Maekawa T, Sumikawa K. High-dose fasudil preserves postconditioning against myocardial infarction under hyperglycemia in rats: role of mitochondrial KATP channels. Cardiovasc Diabetol 2012; 11: 28–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura T, Miki T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ J 2009; 73: 1184–92. [DOI] [PubMed] [Google Scholar]
- 30.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res 2009; 104: 1240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura T, Nishihara M, Miki T. Drug development targeting the glycogen synthase kinase-3β (GSK-3β)-mediated signal transduction pathway: role of GSK-3β in myocardial protection against ischemia/reperfusion injury. J Pharmacol Sci 2009; 109: 162–7. [DOI] [PubMed] [Google Scholar]
- 32.Miki T, Miura T, Hotta H, Tanno M, Yano T, Sato T, Terashima Y, Takada A, Ishikawa S, Shimamoto K. Endoplasmic reticulum stress in diabetic hearts abolishes erythropoietin-induced myocardial protection by impairment of phospho-glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeability transition. Diabetes 2009; 58: 2863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin X, Zheng Y, Zhai X, Zhao X, Cai L. Diabetic inhibition of preconditioning- and postconditioning-mediated myocardial protection against ischemia/reperfusion injury. Exp Diabetes Res 2012; 2012: 198048–198048. [DOI] [PMC free article] [PubMed] [Google Scholar]