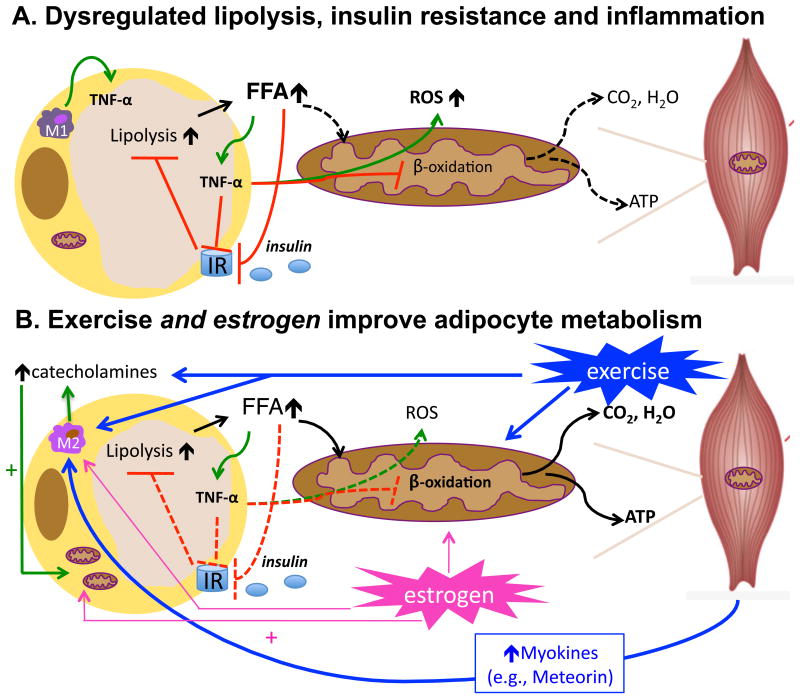
Figure 1. Proposed mechanism by which exercise reduces adipose tissue inflammation.
Depicted in A., adipocyte hypertrophy, as occurs in obesity, leads to dysregulated adipocyte lipolysis and insulin resistance. Insulin's ability to suppress lipolysis becomes impaired leading to increased free fatty acid (FFA) mobilization, even in the presence of insulin. In the absence of FFA utilization, FFAs trigger adipocyte inflammation and an increase in inflammatory (M1) Mϕs which also produce TNF-α, a cytokine that inhibits insulin signaling and impairs mitochondrial function (12) leading to increased reactive oxygen species (ROS) production; ROS further contribute to inflammation, leading to a vicious cycle. Depicted in B., exercise stimulates adipocyte lipolysis via catecholamine activation while also stimulating FFA oxidation in skeletal muscle. Exercise training also reduces adipocyte size, enhances adipocyte insulin sensitivity, and may also stimulate mitochondrial function in adipocytes thereby further limiting the inflammatory effects of FFA within the adipocyte. In addition, exercise triggers the release of myokines, which are cytokines and other peptides released by skeletal muscle. One such recently discovered myokine, meteorin-like, enhances adipocyte insulin sensitivity and reduces adipoctye inflammation (24). Meteorin-like also promotes alternative (M2) Mϕ activation, which is characteristic of healthy insulin-sensitive adipocytes. Similar to exercise, estrogen improves adipocyte metabolism by facilitating FFA oxidation and mitochondrial biogenesis and also via activation of M2 Mϕ, which increases local catecholamine production. Dashed lines = inactive processes; solid lines = active processes.