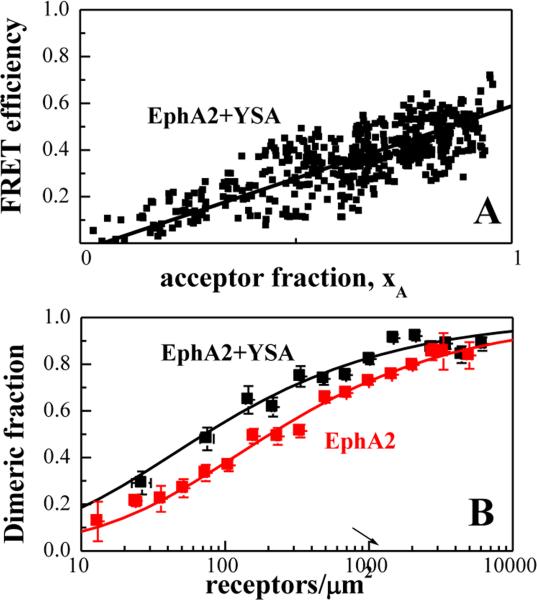
Figure 3. The YSA peptide promotes EphA2 dimerization.
(A) FRET efficiency as a function of receptor acceptor fraction, for total EphA2 concentrations that exceed 2,000 receptors/μm2. Under these conditions, the FRET efficiency depends primarily on the acceptor fraction (xA) and the linear dependence suggests that the oligomer type is a dimer(53-55). (B) Dimerization curves for YSA-bound EphA2 are compared to previously published dimerization curves for unliganded EphA2(39). The dimeric fractions are binned, and averages and standard errors are shown for each bin. The solid line, given by , is the theoretical curve for the best-fit dimerization model. YSA binding stabilizes the EphA2 dimer by −0.7 ± 0.3 kcal/mole.